Inhibition of the rapid component of the delayed rectifier potassium current in ventricular myocytes by angiotensin II via the AT1 receptor
- PMID: 18414380
- PMCID: PMC2442448
- DOI: 10.1038/bjp.2008.95
Inhibition of the rapid component of the delayed rectifier potassium current in ventricular myocytes by angiotensin II via the AT1 receptor
Abstract
Background and purpose: There is increasing evidence that angiotensin II (Ang II) is associated with the occurrence of ventricular arrhythmias. However, little is known about the electrophysiological effects of Ang II on ventricular repolarization. The rapid component of the delayed rectifier K(+) current (I(Kr)) plays a critical role in cardiac repolarization. Hence, the aim of this study was to assess the effect of Ang II on I(Kr) in guinea-pig ventricular myocytes.
Experimental approach: The whole-cell patch-clamp technique was used to record I(Kr) in native cardiocytes and in human embryonic kidney (HEK) 293 cells, co-transfected with human ether-a-go-go-related gene (hERG) encoding the alpha-subunit of I(Kr) and the human Ang II type 1 (AT(1)) receptor gene.
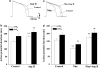
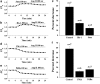
Key results: Ang II decreased the amplitude of I(Kr) in a concentration-dependent manner with an IC(50) of 8.9 nM. Action potential durations at 50% (APD(50)) and 90% (APD(90)) repolarization were prolonged 20% and 16%, respectively by Ang II (100 nM). Ang II-induced inhibition of the I(Kr) was abolished by the AT(1) receptor blocker, losartan (1 muM). Ang II decreased hERG current in HEK293 cells and significantly delayed channel activation, deactivation and recovery from inactivation. Moreover, PKC inhibitors, stausporine and Bis-1, significantly attenuated Ang II-induced inhibition of I(Kr).
Conclusions and implications: Ang II produces an inhibitory effect on I(Kr)/hERG currents via AT(1) receptors linked to the PKC pathway in ventricular myocytes. This is a potential mechanism by which elevated levels of Ang II are involved in the occurrence of arrhythmias in cardiac hypertrophy and failure.
Figures






References
-
- Ahmmed GU, Dong PH, Song G, Ball NA, Xu Y, Walsh RA, et al. Changes in Ca(2+) cycling proteins underlie cardiac action potential prolongation in a pressure-overloaded guinea pig model with cardiac hypertrophy and failure. Circ Res. 2000;86:558–570. - PubMed
-
- Aiello EA, Cingolani HE. Angiotensin II stimulates cardiac L-type Ca(2+) current by a Ca(2+)- and protein kinase C-dependent mechanism. Am J Physiol Heart Circ Physiol. 2001;280:H1528–H1536. - PubMed
-
- Alberte C, Zipes DP. Use of nonantiarrhythmic drugs for prevention of sudden cardiac death. J Cardiovasc Electrophysiol. 2003;14:S87–S95. - PubMed
-
- Bragat AC, Blumenfeld J, Sealey JE. Effect of high-performance liquid chromatography on plasma angiotensin II measurements in treated and untreated normotensive and hypertensive patients. J Hypertens. 1997;15:459–465. - PubMed
-
- Brooksby P, Robinson PJ, Segal R, Klinger G, Pitt B, Cowley AJ. Effects of losartan and captopril on QT dispersion in elderly patients with heart failure. ELITE Study Group. Lancet. 1999;354:395–396. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

