Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells
- PMID: 18414680
- PMCID: PMC2293335
- DOI: 10.1172/JCI34487
Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells
Abstract
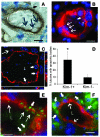
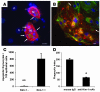
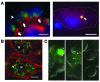
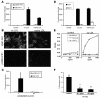
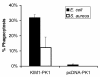
Following injury, the clearance of apoptotic and necrotic cells is necessary for mitigation and resolution of inflammation and tissue repair. In addition to macrophages, which are traditionally assigned to this task, neighboring epithelial cells in the affected tissue are postulated to contribute to this process. Kidney injury molecule-1 (KIM-1 or TIM-1) is an immunoglobulin superfamily cell-surface protein not expressed by cells of the myeloid lineage but highly upregulated on the surface of injured kidney epithelial cells. Here we demonstrate that injured kidney epithelial cells assumed attributes of endogenous phagocytes. Confocal images confirm internalization of apoptotic bodies within KIM-1-expressing epithelial cells after injury in rat kidney tubules in vivo. KIM-1 was directly responsible for phagocytosis in cultured primary rat tubule epithelial cells and also porcine and canine epithelial cell lines. KIM-1 was able to specifically recognize apoptotic cell surface-specific epitopes phosphatidylserine, and oxidized lipoproteins, expressed by apoptotic tubular epithelial cells. Thus, KIM-1 is the first nonmyeloid phosphatidylserine receptor identified to our knowledge that transforms epithelial cells into semiprofessional phagocytes.
Figures



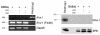
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

