Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice
- PMID: 18414684
- PMCID: PMC2298836
- DOI: 10.1172/JCI34316
Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice
Abstract
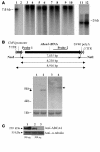
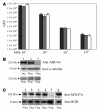
Vectors derived from adeno-associated virus (AAV) are promising for human gene therapy, including treatment for retinal blindness. One major limitation of AAVs as vectors is that AAV cargo capacity has been considered to be restricted to 4.7 kb. Here we demonstrate that vectors with an AAV5 capsid (i.e., rAAV2/5) incorporated up to 8.9 kb of genome more efficiently than 6 other serotypes tested, independent of the efficiency of the rAAV2/5 production process. Efficient packaging of the large murine Abca4 and human MYO7A and CEP290 genes, which are mutated in common blinding diseases, was obtained, suggesting that this packaging efficiency is independent of the specific sequence packaged. Expression of proteins of the appropriate size and function was observed following transduction with rAAV2/5 carrying large genes. Intraocular administration of rAAV2/5 encoding ABCA4 resulted in protein localization to rod outer segments and significant and stable morphological and functional improvement of the retina in Abca4(-/-) mice. This use of rAAV2/5 may be a promising therapeutic strategy for recessive Stargardt disease, the most common form of inherited macular degeneration. The possibility of packaging large genes in AAV greatly expands the therapeutic potential of this vector system.
Figures
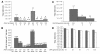

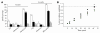
References
-
- Muzyczka, N., and Berns, K.I. 2001. Parvoviridae: the viruses and their replication. Lippincott Williams & Wilkins. Philadelphia, Pennsylvania, USA. 1089–1122.

