Gene-environment interaction in yeast gene expression
- PMID: 18416601
- PMCID: PMC2292755
- DOI: 10.1371/journal.pbio.0060083
Gene-environment interaction in yeast gene expression
Abstract
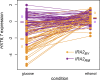
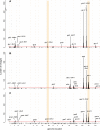
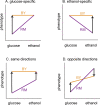
The effects of genetic variants on phenotypic traits often depend on environmental and physiological conditions, but such gene-environment interactions are poorly understood. Recently developed approaches that treat transcript abundances of thousands of genes as quantitative traits offer the opportunity to broadly characterize the architecture of gene-environment interactions. We examined the genetic and molecular basis of variation in gene expression between two yeast strains (BY and RM) grown in two different conditions (glucose and ethanol as carbon sources). We observed that most transcripts vary by strain and condition, with 2,996, 3,448, and 2,037 transcripts showing significant strain, condition, and strain-condition interaction effects, respectively. We expression profiled over 100 segregants derived from a cross between BY and RM in both growth conditions, and identified 1,555 linkages for 1,382 transcripts that show significant gene-environment interaction. At the locus level, local linkages, which usually correspond to polymorphisms in cis-regulatory elements, tend to be more stable across conditions, such that they are more likely to show the same effect or the same direction of effect across conditions. Distant linkages, which usually correspond to polymorphisms influencing trans-acting factors, are more condition-dependent, and often show effects in different directions in the two conditions. We characterized a locus that influences expression of many growth-related transcripts, and showed that the majority of the variation is explained by polymorphism in the gene IRA2. The RM allele of IRA2 appears to inhibit Ras/PKA signaling more strongly than the BY allele, and has undergone a change in selective pressure. Our results provide a broad overview of the genetic architecture of gene-environment interactions, as well as a detailed molecular example, and lead to key insights into how the effects of different classes of regulatory variants are modulated by the environment. These observations will guide the design of studies aimed at understanding the genetic basis of complex traits.
Conflict of interest statement
Figures


References
-
- Talmud PJ. Gene-environment interaction and its impact on coronary heart disease risk. Nutr Metab Cardiovasc Dis. 2007;17:148–152. - PubMed
-
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. - PubMed
-
- Ulrich CM, Kampman E, Bigler J, Schwartz SM, Chen C, et al. Colorectal adenomas and the C677T MTHFR polymorphism: evidence for gene-environment interaction. Cancer Epidemiol Biomarkers Prev. 1999;8:659–668. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

