Cardiac resynchronization: insight from experimental and computational models
- PMID: 18417196
- PMCID: PMC2676869
- DOI: 10.1016/j.pbiomolbio.2008.02.024
Cardiac resynchronization: insight from experimental and computational models
Abstract
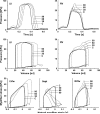
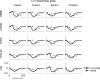
Cardiac resynchronization therapy (CRT) is a promising therapy for heart failure patients with a conduction disturbance, such as left bundle branch block. The aim of CRT is to resynchronize contraction between and within ventricles. However, about 30% of patients do not respond to this therapy. Therefore, a better understanding is needed for the relation between electrical and mechanical activation. In this paper, we focus on to what extent animal experiments and mathematical models can help in order to understand the pathophysiology of asynchrony to further improve CRT.
Figures


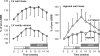



References
-
- Arts T, Delhaas T, Bovendeerd P, Verbeek X, Prinzen FW. Adaptation to mechanical load determines shape and properties of heart and circulation: the CircAdapt model. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H1943–H1954. - PubMed
-
- Auricchio A, Stellbrink C, Block M, Sack S, Vogt J, Bakker P, Klein H, Kramer A, Ding J, Salo R, Tockman B, Pochet T, Spinelli J. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. The Pacing Therapies for Congestive Heart Failure Study Group. The Guidant Congestive Heart Failure Research Group. Circulation. 1999;99:2993–3001. - PubMed
-
- Baller D, Wolpers H-G, Zipfel J, Bretschneider H-J, Hellige G. Comparison of the effects of right atrial, right ventricular apex and atrioventricular sequential pacing on myocardial oxygen consumption and cardiac efficiency: a laboratory investigation. PACE. 1988;11:394–403. - PubMed
-
- Bax JJ, Abraham T, Barold SS, Breithardt OA, Fung JW, Garrigue S, Gorcsan J, 3rd, Hayes DL, Kass DA, Knuuti J, Leclercq C, Linde C, Mark DB, Monaghan MJ, Nihoyannopoulos P, Schalij MJ, Stellbrink C, Yu CM. Cardiac resynchronization therapy: part 1—issues before device implantation. J. Am. Coll. Cardiol. 2005;46:2153–2167. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

