Characterization of the early steps of infection of primary blood monocytes by human immunodeficiency virus type 1
- PMID: 18417568
- PMCID: PMC2447095
- DOI: 10.1128/JVI.02321-07
Characterization of the early steps of infection of primary blood monocytes by human immunodeficiency virus type 1
Abstract
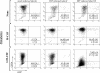
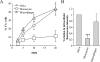
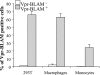
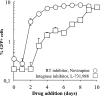
Blood-circulating monocytes migrate in tissues in response to danger stimuli and differentiate there into two major actors of the immune system: macrophages and dendritic cells. Given their migratory behavior and their pivotal role in the orchestration of immune responses, it is not surprising that cells of the monocyte lineage are the target of several viruses, including human immunodeficiency virus type 1 (HIV-1). HIV-1 replicates in monocytoid cells to an extent that is influenced by their differentiation status and modulated by exogenous stimulations. Unstimulated monocytes display a relative resistance to HIV infection mostly exerted during the early steps of the viral life cycle. Despite intensive studies, the identity of the affected step remains controversial, although it is generally assumed to take place after viral entry. We reexamine here the early steps of viral infection of unstimulated monocytes using vesicular stomatitis virus G protein-pseudotyped HIV-1 virions. Our data indicate that a first block to the early steps of infection of monocytes with these particles occurs at the level of viral entry. After entry, reverse transcription and integration proceed with extremely slow kinetics rather than being blocked. Once completed, viral DNA molecules delay entry into the nucleus and integration for up to 5 to 6 days. The inefficacy of these steps accounts for the resistance of monocytes to HIV-1 during the early steps of infection.
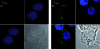
Figures
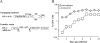
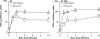
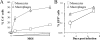
References
-
- Banks, W. A., N. Ercal, and T. O. Price. 2006. The blood-brain barrier in neuroAIDS. Curr. HIV Res. 4259-266. - PubMed
-
- Cavrois, M., C. De Noronha, and W. C. Greene. 2002. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 201151-1154. - PubMed
-
- Clements, J. E., D. H. Gabuzda, and S. L. Gdovin. 1990. Cell type specific and viral regulation of visna virus gene expression. Virus Res. 16175-183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

