Henipavirus V protein association with Polo-like kinase reveals functional overlap with STAT1 binding and interferon evasion
- PMID: 18417573
- PMCID: PMC2447080
- DOI: 10.1128/JVI.00409-08
Henipavirus V protein association with Polo-like kinase reveals functional overlap with STAT1 binding and interferon evasion
Abstract
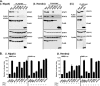
Emerging viruses in the paramyxovirus genus Henipavirus evade host antiviral responses via protein interactions between the viral V and W proteins and cellular STAT1 and STAT2 and the cytosolic RNA sensor MDA5. Polo-like kinase (PLK1) is identified as being an additional cellular partner that can bind to Nipah virus P, V, and W proteins. For both Nipah virus and Hendra virus, contact between the V protein and the PLK1 polo box domain is required for V protein phosphorylation. Results indicate that PLK1 is engaged by Nipah virus V protein amino acids 100 to 160, previously identified as being the STAT1 binding domain responsible for host interferon (IFN) signaling evasion, via a Thr-Ser-Ser-Pro motif surrounding residue 130. A distinct Ser-Thr-Pro motif surrounding residue 199 mediates the PLK1 interaction with Hendra virus V protein. Select mutations in the motif surrounding residue 130 also influenced STAT1 binding and innate immune interference, and data indicate that the V:PLK1 and V:STAT complexes are V mediated yet independent of one another. The effects of STAT1/PLK1 binding motif mutations on the function the Nipah virus P protein in directing RNA synthesis were tested. Remarkably, mutations that selectively disrupt the STAT or PLK1 interaction site have no effects on Nipah virus P protein-mediated viral RNA synthesis. Therefore, mutations targeting V protein-mediated IFN evasion will not alter the RNA synthetic capacity of the virus, supporting an attenuation strategy based on disrupting host protein interactions.
Figures








References
-
- Aaronson, D. S., and C. M. Horvath. 2002. A road map for those who don't know JAK-STAT. Science 2961653-1655. - PubMed
-
- Anonymous. 2004. Hendra virus—Australia (Queensland). ProMED archive number 20041214.3307. International Society for Infectious Diseases, Brookline, MA.
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72248-254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

