Antibody is critical for the clearance of murine norovirus infection
- PMID: 18417579
- PMCID: PMC2447077
- DOI: 10.1128/JVI.00141-08
Antibody is critical for the clearance of murine norovirus infection
Abstract
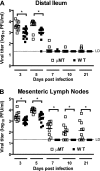
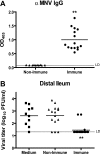
Human noroviruses cause more than 90% of epidemic nonbacterial gastroenteritis. However, the role of B cells and antibody in the immune response to noroviruses is unclear. Previous studies have demonstrated that human norovirus specific antibody levels increase upon infection, but they may not be protective against infection. In this report, we used murine norovirus (MNV), an enteric norovirus, as a model to determine the importance of norovirus specific B cells and immune antibody in clearance of norovirus infection. We show here that mice genetically deficient in B cells failed to clear primary MNV infection as effectively as wild-type mice. In addition, adoptively transferred immune splenocytes derived from B-cell-deficient mice or antibody production-deficient mice were unable to efficiently clear persistent MNV infection in RAG1(-/-) mice. Further, adoptive transfer of either polyclonal anti-MNV serum or neutralizing anti-MNV monoclonal antibodies was sufficient to reduce the level of MNV infection both systemically and in the intestine. Together, these data demonstrate that antibody plays an important role in the clearance of MNV and that immunoglobulin G anti-norovirus antibody can play an important role in clearing mucosal infection.
Figures


References
-
- Ball, J. M., D. Y. Graham, A. R. Opekun, M. A. Gilger, R. A. Guerrero, and M. K. Estes. 1999. Recombinant Norwalk virus-like particles given orally to volunteers: phase I study. Gastroenterology 11740-48. - PubMed
-
- Burstin, S. J., D. R. Spriggs, and B. N. Fields. 1982. Evidence for functional domains on the reovirus type 3 hemagglutinin. Virol. 117146-155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

