Bovine papillomavirus type 1: from clathrin to caveolin
- PMID: 18417596
- PMCID: PMC2447075
- DOI: 10.1128/JVI.00569-08
Bovine papillomavirus type 1: from clathrin to caveolin
Abstract
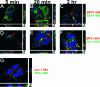
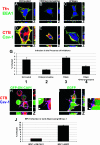
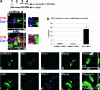
Viruses may infect cells through clathrin-dependent, caveolin-dependent, or clathrin- and caveolin-independent endocytosis. Bovine papillomavirus type 1 (BPV1) entry into cells has been shown to occur by clathrin-dependent endocytosis, a pathway that involves the formation of clathrin-coated pits and fusion to early endosomes. Recently, it has been demonstrated that the closely related JC virus can enter cells in clathrin-coated vesicles and subsequently traffic to caveolae, the organelle where vesicles of the caveolin-dependent pathway deliver their cargo. In this study, we use immunofluorescence staining of BPV1 pseudovirions to show that BPV1 overlaps with the endosome marker EEA1 early during infection and later colocalizes with caveolin-1. We provide evidence through the colocalization of BPV1 with transferrin and cholera toxin B that BPVl trafficking may not be restricted to the clathrin-dependent pathway. Disrupting the entry of caveolar vesicles did not affect BPV1 infection; however, we show that blocking the caveolar pathway postentry results in a loss of BPV1 infection. These data indicate that BPV1 may enter by clathrin-mediated endocytosis and then utilize the caveolar pathway for infection, a pattern of trafficking that may explain the slow kinetics of BPV1 infection.
Figures


Similar articles
-
Clathrin and caveolin-1 expression in primary pigmented rabbit conjunctival epithelial cells: role in PLGA nanoparticle endocytosis.Mol Vis. 2003 Oct 15;9:559-68. Mol Vis. 2003. PMID: 14566223
-
Internalization of cholera toxin by different endocytic mechanisms.J Cell Sci. 2001 Oct;114(Pt 20):3737-47. doi: 10.1242/jcs.114.20.3737. J Cell Sci. 2001. PMID: 11707525
-
Selective caveolin-1-dependent endocytosis of glycosphingolipids.Mol Biol Cell. 2003 Aug;14(8):3254-65. doi: 10.1091/mbc.e02-12-0809. Epub 2003 May 18. Mol Biol Cell. 2003. PMID: 12925761 Free PMC article.
-
Caveosomes and endocytosis of lipid rafts.J Cell Sci. 2003 Dec 1;116(Pt 23):4707-14. doi: 10.1242/jcs.00840. J Cell Sci. 2003. PMID: 14600257 Review.
-
Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms.Traffic. 2003 Nov;4(11):724-38. doi: 10.1034/j.1600-0854.2003.00128.x. Traffic. 2003. PMID: 14617356 Review.
Cited by
-
Hijacking the endocytic machinery by microbial pathogens.Protoplasma. 2010 Aug;244(1-4):75-90. doi: 10.1007/s00709-010-0164-2. Epub 2010 Jun 25. Protoplasma. 2010. PMID: 20574860 Review.
-
Glutathione-conjugate transport by RLIP76 is required for clathrin-dependent endocytosis and chemical carcinogenesis.Mol Cancer Ther. 2011 Jan;10(1):16-28. doi: 10.1158/1535-7163.MCT-10-0699. Mol Cancer Ther. 2011. PMID: 21220488 Free PMC article.
-
From touchdown to transcription: the reovirus cell entry pathway.Curr Top Microbiol Immunol. 2010;343:91-119. doi: 10.1007/82_2010_32. Curr Top Microbiol Immunol. 2010. PMID: 20397070 Free PMC article. Review.
-
Caveolin-1-dependent infectious entry of human papillomavirus type 31 in human keratinocytes proceeds to the endosomal pathway for pH-dependent uncoating.J Virol. 2008 Oct;82(19):9505-12. doi: 10.1128/JVI.01014-08. Epub 2008 Jul 30. J Virol. 2008. PMID: 18667513 Free PMC article.
-
Usage of heparan sulfate, integrins, and FAK in HPV16 infection.Virology. 2010 Jul 20;403(1):1-16. doi: 10.1016/j.virol.2010.04.007. Epub 2010 May 2. Virology. 2010. PMID: 20441998 Free PMC article.
References
-
- Berry, J. M., J. M. Palefsky, and M. L. Welton. 2004. Anal cancer and its precursors in HIV-positive patients: perspectives and management. Surg. Oncol. Clin. N. Am. 13355-373. - PubMed
-
- Bosch, F. X., X. Castellsague, N. Munoz, S. de Sanjose, A. M. Ghaffari, L. C. Gonzalez, M. Gili, I. Izarzugaza, P. Viladiu, C. Navarro, A. Vergara, N. Ascunce, E. Guerrero, and K. V. Shah. 1996. Male sexual behavior and human papillomavirus DNA: key risk factors for cervical cancer in Spain. J. Natl. Cancer Inst. 881060-1067. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

