Interlaboratory comparison of results of an anthrax lethal toxin neutralization assay for assessment of functional antibodies in multiple species
- PMID: 18417668
- PMCID: PMC2446612
- DOI: 10.1128/CVI.00003-08
Interlaboratory comparison of results of an anthrax lethal toxin neutralization assay for assessment of functional antibodies in multiple species
Abstract
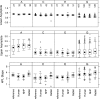
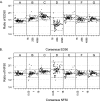
The anthrax lethal toxin neutralization assay (TNA) will likely be used to correlate the protection offered by new anthrax vaccines in animal models to the immunogenicity that will be provided in humans. TNA data are being generated in several different laboratories to measure the immune responses in rabbits, nonhuman primates, and humans. In order to compare data among species and laboratories, a collaborative study was conducted in which 108 samples from the three species were analyzed in seven independent laboratories. Six of the seven laboratories had participated in an interlaboratory technology transfer of the TNA. Analysis of the titration curves generated by samples from each species indicated that the behaviors of the samples from all species were similar; the upper and lower asymptotes and the slopes of the curves were less than 30% divergent from those for human reference material. Dilutional linearity was consistent among samples from each species, with spike to effective dilution at 50% inhibition (ED(50)) slopes of less than 1.2 for all species. Agreement among the laboratories with consensus values was within 10% of the ED(50)s for all samples and within 7.5% of the quotients of the test sample ED(50) and the reference standard ED(50) (NF(50)s) for all samples. The relative standard deviations obtained when data from all laboratories and for all species were combined were 45% for the ED(50)s and 35% for the NF(50)s. These precision data suggest that the NF(50) readout may normalize the values generated by different laboratories. This study demonstrates that the TNA is a panspecies assay that can be performed in several different laboratories with a high degree of quantitative agreement and precision.
Figures



References
-
- Burnham, K. P., and D. R. Anderson. 2002. Model selection and multimodel inference: a practical information-theoretical approach, 2nd. Springer-Verlag, New York, NY.
-
- Campbell, J. D., K. H. Clement, S. S. Wasserman, S. Donegan, L. Chrisley, and K. L. Kotloff. 2007. Safety, reactogenicity and immunogenicity of a recombinant protective antigen anthrax vaccine given to healthy adults. Hum. Vaccines 3:205-211. - PubMed
-
- Gorse, G. J., W. Keitel, H. Keyserling, D. N. Taylor, M. Lock, K. Alves, J. Kenner, L. Deans, and M. Gurwith. 2006. Immunogenicity and tolerance of ascending doses of a recombinant protective antigen (rPA102) anthrax vaccine: a randomized, double-blinded, controlled, multicenter trial. Vaccine 24:5950-5959. - PubMed
-
- Hauck, W. W., R. C. Capen, J. D. Callahan, J. E. De Muth, H. Hsu, D. Lansky, N. C. Sajjadi, S. S. Seaver, R. R. Singer, and D. Weisman. 2005. Assessing parallelism prior to determining relative potency. PDA J. Pharm. Sci. Technol. 59:127-137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

