Silencing the Kir4.1 potassium channel subunit in satellite glial cells of the rat trigeminal ganglion results in pain-like behavior in the absence of nerve injury
- PMID: 18417695
- PMCID: PMC2533133
- DOI: 10.1523/JNEUROSCI.5053-07.2008
Silencing the Kir4.1 potassium channel subunit in satellite glial cells of the rat trigeminal ganglion results in pain-like behavior in the absence of nerve injury
Abstract
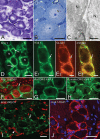
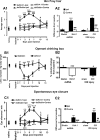
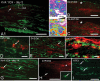
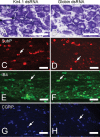
Growing evidence suggests that changes in the ion buffering capacity of glial cells can give rise to neuropathic pain. In the CNS, potassium ion (K+) buffering is dependent on the glia-specific inward rectifying K+ channel Kir4.1. We recently reported that the satellite glial cells that surround primary sensory neurons located in sensory ganglia of the peripheral nervous system also express Kir4.1, whereas the neurons do not. In the present study, we show that, in the rat trigeminal ganglion, the location of the primary sensory neurons for face sensation, specific silencing of Kir4.1 using RNA interference leads to spontaneous and evoked facial pain-like behavior in freely moving rats. We also show that Kir4.1 in the trigeminal ganglion is reduced after chronic constriction injury of the infraorbital nerve. These findings suggests that neuropathic pain can result from a change in expression of a single K+ channel in peripheral glial cells, raising the possibility of targeting Kir4.1 to treat pain in general and particularly neuropathic pain that occurs in the absence of nerve injury.
Figures



References
-
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. - PubMed
-
- Banati RB. Brain plasticity and microglia: is transsynaptic glial activation in the thalamus after limb denervation linked to cortical plasticity and central sensitisation? J Physiol (Paris) 2002;96:289–299. - PubMed
-
- Bhargava A, Dallman MF, Pearce D, Choi S. Long double-stranded RNA-mediated RNA interference as a tool to achieve site-specific silencing of hypothalamic neuropeptides. Brain Res Brain Res Protoc. 2004;13:115–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
