Endotoxin mediates recruitment of RNA polymerase II to target genes in acute renal failure
- PMID: 18417719
- PMCID: PMC2440304
- DOI: 10.1681/ASN.2007121368
Endotoxin mediates recruitment of RNA polymerase II to target genes in acute renal failure
Abstract
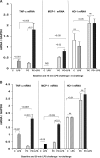
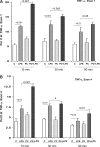
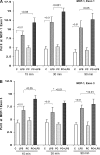
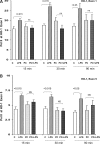
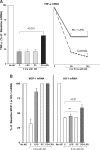
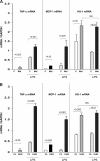
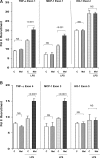
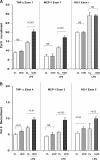
Acute renal failure (ARF) sensitizes the kidney to endotoxin (LPS)-driven production of cytokines and chemokines. This study assessed whether this LPS hyperresponsiveness exists at the genomic level. Three heterogeneous mouse models of ARF were studied: Maleate nephrotoxicity, unilateral ureteral obstruction, and LPS preconditioning. In all cases, LPS was injected approximately 18 h after injury was induced, and over the next 0 to 90 min, RNA polymerase II recruitment to the genome at three LPS-responsive genes (TNF-alpha, monocyte chemoattractant-1 [MCP-1], and heme oxygenase-1 [HO-1]) was assessed by chromatin immunoprecipitation. LPS hyperresponsiveness was noted in each model, measured by exaggerated increases in TNF-alpha and MCP-1 mRNA (approximately two to 10 times higher than LPS-injected controls). Corresponding increases in the recruitment of RNA polymerase II to the TNF-alpha and MCP-1 genes were observed, and increased trimethylation of histone 3 lysine 4 (H3K4m3) at these sites may have played a role in this recruitment. Conversely, recruitment of RNA polymerase II to the HO-1 gene was suppressed ("tolerance"), and no increase in H3K4m3 was observed at HO-1 exons. The ARF-induced changes in mRNA did not correlate with mRNA stability, suggesting the mechanistic importance of RNA polymerase II-mediated transcriptional events. In conclusion, LPS hyperresponsiveness after ARF is likely mediated at the genomic level, possibly by H3K4m3.
Figures
References
-
- Honda N, Hishida A, Ikuma K, Yonemura K: Acquired resistance to acute renal failure. Kidney Int 31: 1233–1238, 1987 - PubMed
-
- Elliott WC, Houghton DC, Gilbert DN, Baines-Hunter J, Bennett WM: Gentamicin nephrotoxicity. I. Degree and permanence of acquired insensitivity. J Lab Clin Med 100: 501–512, 1982 - PubMed
-
- Zager RA, Baltes LA, Sharma HM, Jurkowitz MS: Responses of the ischemic acute renal failure kidney to additional ischemic events. Kidney Int 26: 689–700, 1984 - PubMed
-
- Zager RA, Johnson AC, Hanson SY, Lund S: Ischemic proximal tubular injury primes mice to endotoxin-induced TNF-α generation and systemic release. Am J Physiol 289: F289–F297, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

