Greater epoetin alfa responsiveness is associated with improved survival in hemodialysis patients
- PMID: 18417744
- PMCID: PMC2440273
- DOI: 10.2215/CJN.04601007
Greater epoetin alfa responsiveness is associated with improved survival in hemodialysis patients
Abstract
Background and objectives: Among hemodialysis patients, achieved hemoglobin is associated with Epoetin alfa dose and erythropoietin responsiveness. A prospective erythropoietin responsiveness measure was developed and its association with mortality evaluated.
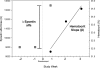
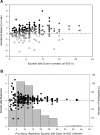
Design, setting, participants, & measurements: Data from 321 participants were used and randomized to the hematocrit normalization arm of the Normal Hematocrit Cardiac Trial. Subjects were to receive a 50% Epoetin alfa dose increase at randomization. The prospective erythropoietin responsiveness measure was defined as the ratio of weekly hematocrit change (over the 3 wk after randomization) per Epoetin alfa dose increase (1000 IU/wk) corresponding to the mandated 50% dose increase at randomization. The distribution of responsiveness was divided into quartiles. Over a 1-yr follow-up, Cox proportional hazard modeling evaluated associations between this responsiveness measure and mortality.
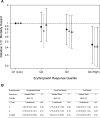
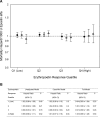
Results: Erythropoietin responsiveness values ranged from -2.1% to 2.4% per week per 1000 IU. Although subjects were similar across response quartiles, mortality ranged between 14% and 34% among subjects in the highest and lowest response quartiles (P = 0.0004), respectively. After adjusting for baseline prognostic indicators, highest versus lowest responsiveness was associated with a hazard ratio of 0.41 (95% confidence interval, 0.20 to 0.87).
Conclusion: Lower erythropoietin responsiveness is a strong, independent predictor of mortality risk and should be considered when evaluating associations between clinical outcomes and potential prognostic indicators, such as Epoetin alfa dose and achieved hemoglobin values.
Figures
Comment in
-
Erythropoietin stimulating agents and epoetin alfa revisited: what's really relevant?Clin J Am Soc Nephrol. 2008 Jul;3(4):935-7. doi: 10.2215/CJN.02300508. Epub 2008 Jun 25. Clin J Am Soc Nephrol. 2008. PMID: 18579671 No abstract available.
References
-
- KDOQI: Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am J Kidney Dis 47[Suppl]: 111–145, 2006 - PubMed
-
- Collins AJ, Brenner RM, Ofman JJ, Chi EM, Stuccio-White N, Krishnan M, Solid C, Ofsthun NJ, Lazarus JM: Epoetin alfa use in patients with ESRD: an analysis of recent US prescribing patterns and hemoglobin outcomes. Am J Kidney Dis 46: 481–488, 2005 - PubMed
-
- Barany P, vino Filho JC, Bergstrom J: High C-reactive protein is a strong predictor of resistance to erythropoietin in hemodialysis patients. Am J Kidney Dis 29: 565–568, 1997 - PubMed
-
- Del VL, Pozzoni P, Andrulli S, Locatelli F: Inflammation and resistance to treatment with recombinant human erythropoietin. J Renal Nutr 15: 137–141, 2005 - PubMed
-
- Hsu SP, Peng YS, Pai MF, Hung KY, Tsai TJ: Influence of relative hypoparathyroidism on the responsiveness to recombinant human erythropoietin in hemodialysis patients. Blood Purif 21: 220–224, 2003 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

