Pharmacogenetic pathway analysis of irinotecan
- PMID: 18418374
- PMCID: PMC2759399
- DOI: 10.1038/clpt.2008.63
Pharmacogenetic pathway analysis of irinotecan
Abstract
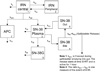
Irinotecan, a chemotherapeutic agent against various solid tumors, is a prodrug requiring activation to SN-38. Irinotecan's complex pharmacokinetics potentially allow for many genetic sources of variability. We explored relationships between pharmacokinetic pathways and polymorphisms in genes associated with irinotecan's metabolism and transport. We fitted a seven-compartment pharmacokinetic model with enterohepatic recirculation (EHR) to concentrations of irinotecan and metabolites SN-38, SN-38 glucuronide (SN-38G), and aminopentanoic acid (APC). Principal component analysis (PCA) of patient-specific parameter estimates produced measures interpretable along pathways. Nine principal components provided good characterization of the overall variation. Polymorphisms in genes UGT1A1, UGT1A7, and UGT1A9 had strong associations with a component corresponding to the irinotecan-to-SN-38 pathway and SN-38 recirculation and to a component relating to SN-38-to-SN-38G conversion and elimination of SN-38G. The component characterizing irinotecan's compartments was associated with HNF1alpha and ABCC2 polymorphisms. The exploratory analysis with PCA in this pharmacogenetic analysis was able to identify known associations and may have allowed identification of previously uncharacterized functional polymorphisms.
Figures



References
-
- Rivera F, Vega-Villegas ME, Lopez-Brea MF. Chemotherapy of advanced gastric cancer. Cancer Treat Rev. 2007;33:315–324. - PubMed
-
- Markman M. New, expanded, and modified use of approved antineoplastic agents in ovarian cancer. Oncologist. 2007;12:186–190. - PubMed
-
- MacCallum C, Gillenwater HH. Second-line treatment of small-cell lung cancer. Curr Oncol Rep. 2006;8:258–264. - PubMed
-
- Kumar A, Wakelee H. Second-and third-line treatments in non-small cell lung cancer. Curr Treat Options Oncol. 2006;7:37–49. - PubMed
-
- Garcia-Carbonero R, Supko JG. Current perspectives on the clinical experience, pharmacology, and continued development of the camptothecins. Clin Cancer Res. 2002;8:641–661. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

