Distribution and neurochemical identification of pancreatic afferents in the mouse
- PMID: 18418900
- PMCID: PMC2677067
- DOI: 10.1002/cne.21736
Distribution and neurochemical identification of pancreatic afferents in the mouse
Abstract
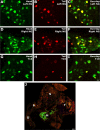
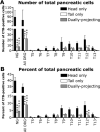
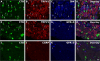
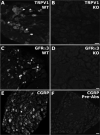
Dysfunction of primary afferents innervating the pancreas has been shown to contribute to the development of painful symptoms during acute and chronic pancreatitis. To investigate the distribution and neurochemical phenotype of pancreatic afferents, Alexa Fluor-conjugated cholera toxin B (CTB) was injected into the pancreatic head (CTB-488) and tail (CTB-555) of adult male mice to label neurons retrogradely in both the dorsal root ganglia (DRG) and nodose ganglia (NG). The NG and DRG (T5-T13) were processed for fluorescent immunohistochemistry and visualized by using confocal microscopy. Spinal pancreatic afferents were observed from T5 to T13, with the greatest contribution coming from T9-T12. The pancreatic afferents were equally distributed between right and left spinal ganglia; however, the innervation from the left NG was significantly greater than from the right. For both spinal and vagal afferents there was significantly greater innervation of the pancreatic head relative to the tail. The total number of retrogradely labeled afferents in the nodose was very similar to the total number of DRG afferents. The neurochemical phenotype of DRG neurons was dominated by transient receptor potential vanilloid 1 (TRPV1)-positive neurons (75%), GDNF family receptor alpha-3 (GFRalpha3)-positive neurons (67%), and calcitonin gene-related peptide (CGRP)-positive neurons(65%) neurons. In the NG, TRPV1-, GFRalpha3-, and CGRP-positive neurons constituted only 35%, 1%, and 15% of labeled afferents, respectively. The disparity in peptide and receptor expression between pancreatic afferents in the NG and DRG suggests that even though they contribute a similar number of primary afferents to the pancreas, these two populations may differ in regard to their nociceptive properties and growth factor dependency.
(c) 2008 Wiley-Liss, Inc.
Figures
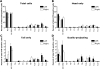

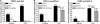
References
-
- Bahns E, Halsband U, Janig W. Responses of sacral visceral afferents from the lower urinary tract, colon and anus to mechanical stimulation. Pflugers Arch. 1987;410:296–303. - PubMed
-
- Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger KS, Leitner ML, Araki T, Johnson EM, Jr, Milbrandt J. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3-RET receptor complex. Neuron. 1998;21:1291–1302. - PubMed
-
- Bennett DL, Dmietrieva N, Priestley JV, Clary D, McMahon SB. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Lett. 1996;206:33–36. - PubMed
-
- Berkley KJ, Hotta H, Robbins A, Sato Y. Functional properties of afferent fibers supplying reproductive and other pelvic organs in pelvic nerve of female rat. J Neurophysiol. 1990;63:256–272. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

