Plasma drug concentrations and virologic evaluations after stopping treatment with nonnucleoside reverse-transcriptase inhibitors in HIV type 1-infected children
- PMID: 18419497
- PMCID: PMC3597130
- DOI: 10.1086/587657
Plasma drug concentrations and virologic evaluations after stopping treatment with nonnucleoside reverse-transcriptase inhibitors in HIV type 1-infected children
Abstract
Background: The optimum strategy for stopping treatment with drugs that have different half-lives in a combination regimen to minimize the risk of selecting drug-resistant viruses remains unknown. We evaluated drug concentrations in plasma, human immunodeficiency virus (HIV) load, and development of drug resistance after a planned treatment interruption of a nonnucleoside reverse-transcriptase inhibitor (NNRTI)-containing regimen in HIV type 1-infected children.
Methods: Children with viral loads <50 copies/mL and CD4 cell percentages > or =30% (for children aged 2-6 years) or CD4 cell percentages > or =25% and CD4 cell counts > or =500 cells/microL (for children aged 7-15 years) were randomized to either a planned treatment interruption or to continuous therapy. In the planned treatment interruption arm, either (1) treatment with nevirapine or efavirenz was stopped, and treatment with the remaining drugs was continued for 7-14 days, or (2) nevirapine or efavirenz were replaced by a protease inhibitor, and all drugs were stopped after 7-14 days. Sampling for determination of plasma drug concentrations, measurement of viral load, and drug resistance testing was scheduled at day 0, day 7 (drug concentrations only), day 14, and day 28 after interruption of treatment with an NNRTI.
Results: Treatment with an NNRTI was interrupted for 35 children (20 were receiving nevirapine, and 15 were receiving efavirenz). Median time from NNRTI cessation to stopping all drugs was 9 days (range, 6-15 days) for nevirapine and 14 days (range, 6-18 days) for efavirenz. At 7 days, 1 (5%) of 19 and 4 (50%) of 8 children had detectable nevirapine and efavirenz concentrations, respectively; efavirenz remained detectable in 3 (25%) of 12 children at 14 days. At 14 days, viral load was > or =50 copies/mL in 6 of 16 children interrupting treatment with nevirapine (range, 52-7000 copies/mL) and in 2 of 12 children interrupting treatment with efavirenz (range, 120-1600 copies/mL). No new NNRTI mutations were observed.
Conclusions: In children with virological suppression who experienced interruption of treatment with an NNRTI, staggered or replacement stopping strategies for a median of 9 days for nevirapine and 14 days for efavirenz were not associated with the selection of NNRTI resistance mutations.
Figures
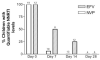
References
-
- Arnedo-Valero M, Garcia F, Gil C, et al. Risk of selecting de novo drug-resistance mutations during structured treatment interruptions in patients with chronic HIV infection. Clin Infect Dis. 2005;41:883–90. - PubMed
-
- Moore KH, Barrett JE, Shaw S, et al. The pharmacokinetics of lamivudine phosphorylation in peripheral blood mononuclear cells from patients infected with HIV-1. AIDS. 1999;13:2239–50. - PubMed
-
- Taylor S, Allen S, Fidler S, et al. Stop study: after discontinuation of efavirenz, plasma concentrations may persist for 2 weeks or longer [abstract 131]; Program and abstracts of the 11th Conference on Retroviruses and Opportunistic Infections; San Francisco, California. 2004.
-
- Kikaire B, Khoo S, Walker AS, et al. Nevirapine clearance from plasma in African adults stopping therapy: a pharmacokinetic substudy. AIDS. 2007;21:733–7. - PubMed
-
- Cressey TR, Jourdain G, Lallemant MJ, et al. Persistence of nevirapine exposure during the postpartum period after intrapartum single-dose nevirapine in addition to zidovudine prophylaxis for the prevention of mother-to-child transmission of HIV-1. J Acquir Immune Defic Syndr. 2005;38:283–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

