Opposite effects of uracil and adenine nucleotides on the survival of murine cardiomyocytes
- PMID: 18419595
- PMCID: PMC3822540
- DOI: 10.1111/j.1582-4934.2007.00133.x
Opposite effects of uracil and adenine nucleotides on the survival of murine cardiomyocytes
Abstract
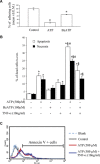
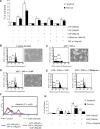
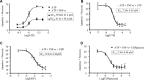
We previously showed that the human heart expresses all known P2X and P2Y receptors activated by extra-cellular adenine or uracil nucleotides. Despite evidence that, both in humans and rodents, plasma levels of ATP and UTP markedly increase during myocardial infarction, the differential effects mediated by the various adenine- and uracil-preferring myocardial P2 receptors are still largely unknown. Here, we studied the effects of adenine and uracil nucleotides on murine HL-1 cardiomyocytes. RT-PCR analysis showed that HL-1 cardiomyocytes express all known P2X receptors (except for P2X(2)), as well as the P2Y(2,4,6,14) subtypes. Exposure of cardiomyocytes to adenine nucleotides (ATP, ADP or BzATP) induced apoptosis and necrosis, as determined by flow-cytometry. Cell death was exacerbated by tumour necrosis factor (TNF)-alpha, a cytokine implicated in chronic heart failure progression. Conversely, uracil nucleotides (UTP, UDP and UDPglucose) had no effect 'per se', but fully counteracted the deleterious effects induced by adenine nucleotides and TNF-alpha, even if added to cardiomyocytes after beginning exposure to these cell death-inducing agents. Thus, exposure of cardiomyocytes to elevated concentrations of ATP or ADP in the presence of TNF-alpha contributes to cell death, an effect which is counteracted by uracil-preferring P2 receptors. Cardiomyocytes do not need to be 'primed' by uracil nucleotides to become insensitive to adenine nucleotides-induced death, suggesting the existence of a possible 'therapeutic' window for uracil nucleotides-mediated protection. Thus, release of UTP during cardiac ischaemia and in chronic heart failure may protect against myocardial damage, setting the basis for developing novel cardioprotective agents that specifically target uracil-preferring P2Y receptors.
Figures



Similar articles
-
Involvement of uracil nucleotides in protection of cardiomyocytes from hypoxic stress.Biochem Pharmacol. 2005 Apr 15;69(8):1215-23. doi: 10.1016/j.bcp.2005.01.018. Biochem Pharmacol. 2005. PMID: 15794942 Free PMC article.
-
Stimulation of mouse cultured sympathetic neurons by uracil but not adenine nucleotides.Neuroscience. 2001;103(1):227-36. doi: 10.1016/s0306-4522(00)00547-9. Neuroscience. 2001. PMID: 11311803
-
Assessment and characterization of purinergic contractions and relaxations in the rat urinary bladder.Basic Clin Pharmacol Toxicol. 2010 Jul;107(1):603-13. doi: 10.1111/j.1742-7843.2010.00554.x. Epub 2010 Apr 12. Basic Clin Pharmacol Toxicol. 2010. PMID: 20406212
-
Molecular recognition at purine and pyrimidine nucleotide (P2) receptors.Curr Top Med Chem. 2004;4(8):805-19. doi: 10.2174/1568026043450961. Curr Top Med Chem. 2004. PMID: 15078212 Free PMC article. Review.
-
P2 receptors activated by uracil nucleotides--an update.Curr Med Chem. 2006;13(3):289-312. doi: 10.2174/092986706775476052. Curr Med Chem. 2006. PMID: 16475938 Review.
Cited by
-
Apyrase treatment of myocardial infarction according to a clinically applicable protocol fails to reduce myocardial injury in a porcine model.BMC Cardiovasc Disord. 2010 Jan 4;10:1. doi: 10.1186/1471-2261-10-1. BMC Cardiovasc Disord. 2010. PMID: 20047685 Free PMC article.
-
Cardiac purinergic signalling in health and disease.Purinergic Signal. 2015 Mar;11(1):1-46. doi: 10.1007/s11302-014-9436-1. Epub 2014 Dec 20. Purinergic Signal. 2015. PMID: 25527177 Free PMC article. Review.
-
Cardiomyocyte death induced by ischaemic/hypoxic stress is differentially affected by distinct purinergic P2 receptors.J Cell Mol Med. 2012 May;16(5):1074-84. doi: 10.1111/j.1582-4934.2011.01382.x. J Cell Mol Med. 2012. PMID: 21762374 Free PMC article.
-
Metabolic reaction fluxes as amplifiers and buffers of risk alleles for coronary artery disease.Mol Syst Biol. 2025 Jun;21(6):676-695. doi: 10.1038/s44320-025-00097-2. Epub 2025 Apr 2. Mol Syst Biol. 2025. PMID: 40175777 Free PMC article.
-
Role of cAMP in Cardiomyocyte Viability: Beneficial or Detrimental?Circ Res. 2023 Nov 10;133(11):902-923. doi: 10.1161/CIRCRESAHA.123.322652. Epub 2023 Oct 18. Circ Res. 2023. PMID: 37850368 Free PMC article.
References
-
- Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. - PubMed
-
- Abbracchio MP, Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64:445–75. - PubMed
-
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–92. - PubMed
-
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–67. - PubMed
-
- Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, Burnstock G. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci. 2003;24:52–5. - PMC - PubMed

