Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes
- PMID: 18419773
- PMCID: PMC2562661
- DOI: 10.1111/j.1462-5822.2008.01160.x
Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes
Abstract
Vesicular secretion of macromolecules has recently been described in the basidiomycete Cryptococcus neoformans, raising the question as to whether ascomycetes similarly utilize vesicles for transport. In the present study, we examine whether the clinically important ascomycete Histoplasma capsulatum produce vesicles and utilized these structures to secrete macromolecules. Transmission electron microscopy (TEM) shows transcellular secretion of vesicles by yeast cells. Proteomic and lipidomic analyses of vesicles isolated from culture supernatants reveal a rich collection of macromolecules involved in diverse processes, including metabolism, cell recycling, signalling and virulence. The results demonstrate that H. capsulatum can utilize a trans-cell wall vesicular transport secretory mechanism to promote virulence. Additionally, TEM of supernatants collected from Candida albicans, Candida parapsilosis, Sporothrix schenckii and Saccharomyces cerevisiae documents that vesicles are similarly produced by additional ascomycetes. The vesicles from H. capsulatum react with immune serum from patients with histoplasmosis, providing an association of the vesicular products with pathogenesis. The findings support the proposal that vesicular secretion is a general mechanism in fungi for the transport of macromolecules related to virulence and that this process could be a target for novel therapeutics.
Conflict of interest statement
Figures


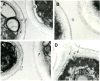

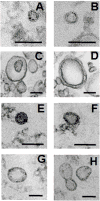

References
-
- Almeida-Paes R, Pimenta MA, Pizzini CV, Monteiro PC, Peralta JM, Nosanchuk JD, Zancope-Oliveira RM. Use of mycelial-phase Sporothrix schenckii exoantigens in an enzyme-linked immunosorbent assay for diagnosis of sporotrichosis by antibody detection. Clin Vaccine Immunol. 2007b;14:244–249. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G12 RR008124/RR/NCRR NIH HHS/United States
- R01 AI033774/AI/NIAID NIH HHS/United States
- R01 AI056070/AI/NIAID NIH HHS/United States
- D43 TW007129/TW/FIC NIH HHS/United States
- HL059842/HL/NHLBI NIH HHS/United States
- R01 AI052733/AI/NIAID NIH HHS/United States
- AI052733/AI/NIAID NIH HHS/United States
- R37 AI033142/AI/NIAID NIH HHS/United States
- 5G12RR008124/RR/NCRR NIH HHS/United States
- R01 AI033142/AI/NIAID NIH HHS/United States
- AI52733/AI/NIAID NIH HHS/United States
- P30 AI051519/AI/NIAID NIH HHS/United States
- R01 HL059842/HL/NHLBI NIH HHS/United States
- AI51519/AI/NIAID NIH HHS/United States
- AI033142/AI/NIAID NIH HHS/United States
- AI033774/AI/NIAID NIH HHS/United States
- D43-TW007129/TW/FIC NIH HHS/United States
- AI056070-01A2/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

