Intracellular localization and subsequent redistribution of metal transporters in a rat choroid plexus model following exposure to manganese or iron
- PMID: 18420243
- PMCID: PMC2586425
- DOI: 10.1016/j.taap.2008.02.024
Intracellular localization and subsequent redistribution of metal transporters in a rat choroid plexus model following exposure to manganese or iron
Abstract
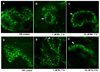
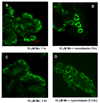
Confocal microscopy was used to investigate the effects of manganese (Mn) and iron (Fe) exposure on the subcellular distribution of metal transporting proteins, i.e., divalent metal transporter 1 (DMT1), metal transporter protein 1 (MTP1), and transferrin receptor (TfR), in the rat intact choroid plexus which comprises the blood-cerebrospinal fluid barrier. In control tissue, DMT1 was concentrated below the apical epithelial membrane, MTP1 was diffuse within the cytosol, and TfR was distributed in vesicles around nuclei. Following Mn or Fe treatment (1 and 10 microM), the distribution of DMT1 was not affected. However, MTP1 and TfR moved markedly toward the apical pole of the cells. These shifts were abolished when microtubules were disrupted. Quantitative RT-PCR and Western blot analyses revealed a significant increase in mRNA and protein levels of TfR but not DMT1 and MTP1 after Mn exposure. These results suggest that early events in the tissue response to Mn or Fe exposure involve microtubule-dependent, intracellular trafficking of MTP1 and TfR. The intracellular trafficking of metal transporters in the choroid plexus following Mn exposure may partially contribute to Mn-induced disruption in Fe homeostasis in the cerebrospinal fluid (CSF) following Mn exposure.
Figures







References
-
- Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275:19906–19912. - PubMed
-
- Andrews NC. The iron transporter DMT1. Int J Biochem Cell Biol. 1999;31:991–994. - PubMed
-
- Aschner M, Vrana KE, Zheng W. Manganese uptake and distribution in the central nervous system (CNS) Neurotoxicology. 1999;20:173–180. - PubMed
-
- Bali PK, Zak O, Aisen P. A new role for the transferrin receptor in the release of iron from transferrin. Biochemistry. 1991;30:324–328. - PubMed
-
- Berg D, Gerlach M, Youdim MB, Double KL, Zecca L, Riederer P, Becker G. Brain iron pathways and their relevance to Parkinson's disease. J Neurochem. 2001;79:225–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

