Coupling between somatosensory evoked potentials and hemodynamic response in the rat
- PMID: 18420425
- PMCID: PMC2486435
- DOI: 10.1016/j.neuroimage.2008.02.061
Coupling between somatosensory evoked potentials and hemodynamic response in the rat
Abstract
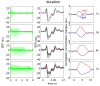
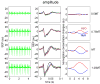
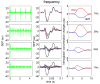
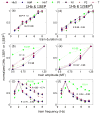
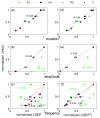
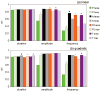
We studied the relationship between somatosensory evoked potentials (SEP) recorded with scalp electroencephalography (EEG) and hemoglobin responses recorded non-invasively with diffuse optical imaging (DOI) during parametrically varied electrical forepaw stimulation in rats. Using these macroscopic techniques we verified that the hemodynamic response is not linearly coupled to the somatosensory evoked potentials, and that a power or threshold law best describes the coupling between SEP and the hemoglobin response, in agreement with the results of most invasive studies. We decompose the SEP response in three components (P1, N1, and P2) to determine which best predicts the hemoglobin response. We found that N1 and P2 predict the hemoglobin response significantly better than P1 and the input stimuli (S). Previous electrophysiology studies reported in the literature show that P1 originates in layer IV directly from thalamocortical afferents, while N1 and P2 originate in layers I and II and reflect the majority of local cortico-cortical interactions. Our results suggest that the evoked hemoglobin response is driven by the cortical synaptic activity and not by direct thalamic input. The N1 and P2 components, and not P1, need to be considered to correctly interpret neurovascular coupling.
Figures
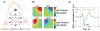

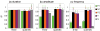
References
-
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. - PubMed
-
- Allison T, McCarthy G, Wood CC, Darcey TM, Spencer DD, Williamson PD. Human cortical potentials evoked by stimulation of the median nerve. I. Cytoarchitectonic areas generating short-latency activity. J Neurophysiol. 1989a;62:694–710. - PubMed
-
- Allison T, McCarthy G, Wood CC, Williamson PD, Spencer DD. Human cortical potentials evoked by stimulation of the median nerve. II. Cytoarchitectonic areas generating long-latency activity. J Neurophysiol. 1989b;62:711–722. - PubMed
-
- Ances BM, Zarahn E, Greenberg JH, Detre JA. Coupling of neural activation to blood flow in the somatosensory cortex of rats is time-intensity separable, but not linear. J Cereb Blood Flow Metab. 2000;20:921–930. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

