Weekly paclitaxel in the adjuvant treatment of breast cancer
- PMID: 18420499
- PMCID: PMC2743943
- DOI: 10.1056/NEJMoa0707056
Weekly paclitaxel in the adjuvant treatment of breast cancer
Erratum in
- N Engl J Med. 2008 Jul 3;359(1):106
- N Engl J Med. 2009 Apr 16;360(16):1685
Abstract
Background: We compared the efficacy of two different taxanes, docetaxel and paclitaxel, given either weekly or every 3 weeks, in the adjuvant treatment of breast cancer.
Methods: We enrolled 4950 women with axillary lymph node-positive or high-risk, lymph node-negative breast cancer. After randomization, all patients first received 4 cycles of intravenous doxorubicin and cyclophosphamide at 3-week intervals and were then assigned to intravenous paclitaxel or docetaxel given at 3-week intervals for 4 cycles or at 1-week intervals for 12 cycles. The primary end point was disease-free survival.
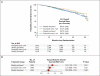
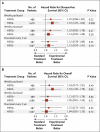
Results: As compared with patients receiving standard therapy (paclitaxel every 3 weeks), the odds ratio for disease-free survival was 1.27 among those receiving weekly paclitaxel (P=0.006), 1.23 among those receiving docetaxel every 3 weeks (P=0.02), and 1.09 among those receiving weekly docetaxel (P=0.29) (with an odds ratio >1 favoring the groups receiving experimental therapy). As compared with standard therapy, weekly paclitaxel was also associated with improved survival (odds ratio, 1.32; P=0.01). An exploratory analysis of a subgroup of patients whose tumors expressed no human epidermal growth factor receptor type 2 protein found similar improvements in disease-free and overall survival with weekly paclitaxel treatment, regardless of hormone-receptor expression. Grade 2, 3, or 4 neuropathy was more frequent with weekly paclitaxel than with paclitaxel every 3 weeks (27% vs. 20%).
Conclusions: Weekly paclitaxel after standard adjuvant chemotherapy with doxorubicin and cyclophosphamide improves disease-free and overall survival in women with breast cancer. (ClinicalTrials.gov number, NCT00004125 [ClinicalTrials.gov].).
Copyright 2008 Massachusetts Medical Society.
Figures


Comment in
-
Weekly paclitaxel in the adjuvant treatment of breast cancer.N Engl J Med. 2008 Jul 17;359(3):310; author reply 310-1. doi: 10.1056/NEJMc081136. N Engl J Med. 2008. PMID: 18635438 No abstract available.
-
Weekly paclitaxel in the adjuvant treatment of breast cancer.N Engl J Med. 2008 Jul 17;359(3):310; author reply 310-1. N Engl J Med. 2008. PMID: 18642411 No abstract available.
-
Choosing a taxane for adjuvant treatment of breast cancer: more than a flip of the coin?Nat Clin Pract Oncol. 2008 Oct;5(10):570-1. doi: 10.1038/ncponc1215. Epub 2008 Sep 2. Nat Clin Pract Oncol. 2008. PMID: 18762793
References
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. - PubMed
-
- Mamounas EP, Bryant J, Lembersk B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23:3686–96. - PubMed
-
- Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–83. - PubMed
-
- Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–13. - PubMed
-
- Sparano JA. Taxanes for breast cancer: an evidence-based review of randomized phase II and phase III trials. Clin Breast Cancer. 2000;1:32–42. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- CA11789/CA/NCI NIH HHS/United States
- CA14958/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA049883/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- U10 CA037403/CA/NCI NIH HHS/United States
- U10 CA016116/CA/NCI NIH HHS/United States
- CA32012/CA/NCI NIH HHS/United States
- CA16116/CA/NCI NIH HHS/United States
- U10 CA014958/CA/NCI NIH HHS/United States
- CA66636/CA/NCI NIH HHS/United States
- CA25224/CA/NCI NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- P30 CA013330/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- U24 CA114737/CA/NCI NIH HHS/United States
- CA23318/CA/NCI NIH HHS/United States
- CA49883/CA/NCI NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
