Biological roles for the NOX family NADPH oxidases
- PMID: 18420576
- PMCID: PMC2427363
- DOI: 10.1074/jbc.R700045200
Biological roles for the NOX family NADPH oxidases
Figures
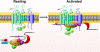
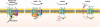
References
-
- Baehner, R. L., and Nathan, D. G. (1967) Science 155 835–836 - PubMed
-
- Suh, Y.-A., Arnold, R. S., Lassegue, B., Shi, J., Xu, X., Sorescu, D., Chung, A. B., Griendling, K. K., and Lambeth, J. D. (1999) Nature 401 79–82 - PubMed
-
- Geiszt, M. (2006) Cardiovasc. Res. 71 289–299 - PubMed
-
- Bedard, K., and Krause, K.-H. (2007) Physiol. Rev. 87 245–313 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

