Targeted ablation of glucose-dependent insulinotropic polypeptide-producing cells in transgenic mice reduces obesity and insulin resistance induced by a high fat diet
- PMID: 18420580
- PMCID: PMC2440595
- DOI: 10.1074/jbc.M710466200
Targeted ablation of glucose-dependent insulinotropic polypeptide-producing cells in transgenic mice reduces obesity and insulin resistance induced by a high fat diet
Abstract
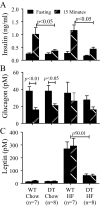
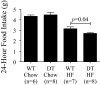
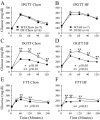
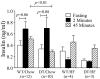
The K cell is a specific sub-type of enteroendocrine cell located in the proximal small intestine that produces glucose-dependent insulinotropic polypeptide (GIP), xenin, and potentially other unknown hormones. Because GIP promotes weight gain and insulin resistance, reducing hormone release from K cells could lead to weight loss and increased insulin sensitivity. However, the consequences of coordinately reducing circulating levels of all K cell-derived hormones are unknown. To reduce the number of functioning K cells, regulatory elements from the rat GIP promoter/gene were used to express an attenuated diphtheria toxin A chain in transgenic mice. K cell number, GIP transcripts, and plasma GIP levels were profoundly reduced in the GIP/DT transgenic mice. Other enteroendocrine cell types were not ablated. Food intake, body weight, and blood glucose levels in response to insulin or intraperitoneal glucose were similar in control and GIP/DT mice fed standard chow. In contrast to single or double incretin receptor knock-out mice, the incretin response was absent in GIP/DT animals suggesting K cells produce GIP plus an additional incretin hormone. Following high fat feeding for 21-35 weeks, the incretin response was partially restored in GIP/DT mice. Transgenic versus wild-type mice demonstrated significantly reduced body weight (25%), plasma leptin levels (77%), and daily food intake (16%) plus enhanced energy expenditure (10%) and insulin sensitivity. Regardless of diet, long term glucose homeostasis was not grossly perturbed in the transgenic animals. In conclusion, studies using GIP/DT mice demonstrate an important role for K cells in the regulation of body weight and insulin sensitivity.
Figures










References
-
- Aiken, K. D., Kisslinger, J. A., and Roth, K. A. (1994) Dev. Dyn. 201 636-670 - PubMed
-
- Sjolund, K., Sanden, G., Hakanson, R., and Sundler, F. (1983) Gastroenterology 85 1120-1130 - PubMed
-
- Brand, S. J., and Schmidt, W. E. (1995) in Textbook of Gastroenterology (Yamada, T., ed) pp. 25-741, JB Lippincott Company, Philadelphia
-
- Walsh, J. H. (1994) in Physiology of the Gastrointestinal Tract (Johnson, L. R., ed) pp. 1-128, Raven Press, New York
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

