Phosphocreatine as an energy source for actin cytoskeletal rearrangements during myoblast fusion
- PMID: 18420707
- PMCID: PMC2517193
- DOI: 10.1113/jphysiol.2008.151027
Phosphocreatine as an energy source for actin cytoskeletal rearrangements during myoblast fusion
Abstract
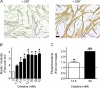
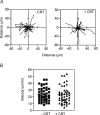
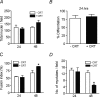
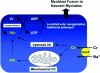
Myoblast fusion is essential for muscle development, postnatal growth and muscle repair after injury. Recent studies have demonstrated roles for actin polymerization during myoblast fusion. Dynamic cytoskeletal assemblies directing cell-cell contact, membrane coalescence and ultimately fusion require substantial cellular energy demands. Various energy generating systems exist in cells but the partitioning of energy sources during myoblast fusion is unknown. Here, we demonstrate a novel role for phosphocreatine (PCr) as a spatiotemporal energy buffer during primary mouse myoblast fusion with nascent myotubes. Creatine treatment enhanced cell fusion in a creatine kinase (CK)-dependent manner suggesting that ATP-consuming reactions are replenished through the PCr/CK system. Furthermore, selective inhibition of actin polymerization prevented myonuclear addition following creatine treatment. As myotube formation is dependent on cytoskeletal reorganization, our findings suggest that PCr hydrolysis is coupled to actin dynamics during myoblast fusion. We conclude that myoblast fusion is a high-energy process, and can be enhanced by PCr buffering of energy demands during actin cytoskeletal rearrangements in myoblast fusion. These findings implicate roles for PCr as a high-energy phosphate buffer in the fusion of multiple cell types including sperm/oocyte, trophoblasts and macrophages. Furthermore, our results suggest the observed beneficial effects of oral creatine supplementation in humans may result in part from enhanced myoblast fusion.
Figures




Comment in
-
The phosphocreatine-creatine kinase system helps to shape muscle cells and keep them healthy and alive.J Physiol. 2008 Jun 15;586(12):2817-8. doi: 10.1113/jphysiol.2008.155358. J Physiol. 2008. PMID: 18556720 Free PMC article. No abstract available.
References
-
- Alfieri RR, Bonelli MA, Cavazzoni A, Brigotti M, Fumarola C, Sestili P, Mozzoni P, De Palma G, Mutti A, Carnicelli D, Vacondio F, Silva C, Borghetti AF, Wheeler KP, Petronini PG. Creatine as a compatible osmolyte in muscle cells exposed to hypertonic stress. J Physiol. 2006;576:391–401. - PMC - PubMed
-
- Belmont LD, Patterson GM, Drubin DG. New actin mutants allow further characterization of the nucleotide binding cleft and drug binding sites. J Cell Sci. 1999;112:1325–1336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

