Prolactin promotes the secretion of active cathepsin D at the basal side of rat mammary acini
- PMID: 18420735
- PMCID: PMC2488222
- DOI: 10.1210/en.2008-0249
Prolactin promotes the secretion of active cathepsin D at the basal side of rat mammary acini
Abstract
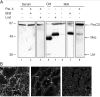
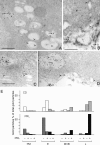
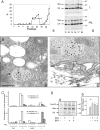
Cathepsin D (CD), a lysosomal aspartic protease present in mammary tissue and milk in various molecular forms, is also found in the incubation medium of mammary acini in molecular forms that are proteolytically active on prolactin at a physiological pH. Because prolactin controls the vesicular traffic in mammary cells, we studied, in vivo and in vitro, its effects on the polarized transport and secretion of various forms of CD in the rat mammary gland. CD accumulated in vesicles not involved in endocytosis in the basal region of cells. Prolactin increased this accumulation and the release of endosomal active single-chain CD at the basal side of acini. The CD-mediated proteolysis of prolactin, leading to the antiangiogenic 16-kDa form, at a physiological pH, was observed only in conditioned medium but not milk. These data support the novel concept that an active molecular form of CD, secreted at the basal side of the mammary epithelium, participates in processing blood-borne prolactin outside the cell, this polarized secretion being controlled by prolactin itself.
Figures





Similar articles
-
Cathepsin D released by lactating rat mammary epithelial cells is involved in prolactin cleavage under physiological conditions.J Cell Sci. 2004 Oct 1;117(Pt 21):5155-64. doi: 10.1242/jcs.01396. J Cell Sci. 2004. PMID: 15456852
-
Brefeldin A differently affects basal and prolactin-stimulated milk protein secretion in lactating rabbit mammary epithelial cells.Eur J Cell Biol. 1997 Apr;72(4):324-36. Eur J Cell Biol. 1997. PMID: 9127732
-
[The lysosomal pathway of prolactin degradation in the mammary gland: kinetics of prolactin hydrolysis by cathepsin D and the peptides formed thereby].Biokhimiia. 1989 Apr;54(4):629-39. Biokhimiia. 1989. PMID: 2758071 Russian.
-
Interplay between progesterone and prolactin in mammary development and implications for breast cancer.Mol Cell Endocrinol. 2012 Jun 24;357(1-2):101-7. doi: 10.1016/j.mce.2011.09.020. Epub 2011 Sep 16. Mol Cell Endocrinol. 2012. PMID: 21945475 Review.
-
Regulation of cell number in the mammary gland by controlling the exfoliation process in milk in ruminants.J Dairy Sci. 2016 Jan;99(1):854-63. doi: 10.3168/jds.2015-9964. J Dairy Sci. 2016. PMID: 26433413 Review.
Cited by
-
Knock-down of cathepsin D affects the retinal pigment epithelium, impairs swim-bladder ontogenesis and causes premature death in zebrafish.PLoS One. 2011;6(7):e21908. doi: 10.1371/journal.pone.0021908. Epub 2011 Jul 1. PLoS One. 2011. PMID: 21747967 Free PMC article.
-
N-terminal prolactin-derived fragments, vasoinhibins, are proapoptoptic and antiproliferative in the anterior pituitary.PLoS One. 2011;6(7):e21806. doi: 10.1371/journal.pone.0021806. Epub 2011 Jul 7. PLoS One. 2011. PMID: 21760910 Free PMC article.
-
Chemotherapy drug response in ovarian cancer cells strictly depends on a cathepsin D-Bax activation loop.J Cell Mol Med. 2009 Jun;13(6):1096-109. doi: 10.1111/j.1582-4934.2008.00435.x. Epub 2008 Jul 24. J Cell Mol Med. 2009. PMID: 18657225 Free PMC article.
-
The role of cathepsins in involution and breast cancer.J Mammary Gland Biol Neoplasia. 2009 Jun;14(2):171-9. doi: 10.1007/s10911-009-9126-8. Epub 2009 May 13. J Mammary Gland Biol Neoplasia. 2009. PMID: 19437107
-
Cathepsin D: Regulation in mammary gland remodeling, misregulation in breast cancer.Cancer Biol Ther. 2010 Sep 1;10(5):467-70. doi: 10.4161/cbt.10.5.12855. Epub 2010 Sep 30. Cancer Biol Ther. 2010. PMID: 20647745 Free PMC article. No abstract available.
References
-
- Freeman ME, Kanyicska B, Lerant A, Nagy G 2000 Prolactin: structure, function and regulation of secretion. Physiol Rev 80:1523–1631 - PubMed
-
- Ollivier-Bousquet M 1978 Early effects of prolactin on lactating rabbit mammary gland. Ultrastructural changes and stimulation of casein secretion. Cell Tissue Res 187:25–43 - PubMed
-
- Ollivier-Bousquet M 1998 Transferrin and prolactin transcytosis in the lactating mammary epithelial cell. J Mammary Gland Biol Neoplasia 3:303–313 - PubMed
-
- Pauloin A, Delpal S, Chanat E, Lavialle F, Aubourg A, Ollivier-Bousquet M 1997 Brefeldin A differently affects basal and prolactin-stimulated milk protein secretion in lactating rabbit mammary epithelial cells. Eur J Cell Biol 72:324–336 - PubMed
-
- Lkhider M, Castino R, Bouguyon E, Isidoro C, Ollivier-Bousquet M 2004 Cathepsin D released by lactating rat mammary epithelial cells is involved in prolactin cleavage under physiological conditions. J Cell Sci 117:5155–5164 - PubMed

