Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc
- PMID: 18420934
- PMCID: PMC2409116
- DOI: 10.1126/science.1154315
Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc
Abstract
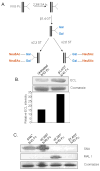
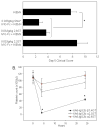
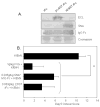
It is well established that high doses of monomeric immunoglobulin G (IgG) purified from pooled human plasma [intravenous immunoglobulin (IVIG)] confer anti-inflammatory activity in a variety of autoimmune settings. However, exactly how those effects are mediated is not clear because of the heterogeneity of IVIG. Recent studies have demonstrated that the anti-inflammatory activity of IgG is completely dependent on sialylation of the N-linked glycan of the IgG Fc fragment. Here we determine the precise glycan requirements for this anti-inflammatory activity, allowing us to engineer an appropriate IgG1 Fc fragment, and thus generate a fully recombinant, sialylated IgG1 Fc with greatly enhanced potency. This therapeutic molecule precisely defines the biologically active component of IVIG and helps guide development of an IVIG replacement with improved activity and availability.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

