Surfactant proteins A and D suppress alveolar macrophage phagocytosis via interaction with SIRP alpha
- PMID: 18420961
- PMCID: PMC2453510
- DOI: 10.1164/rccm.200711-1661OC
Surfactant proteins A and D suppress alveolar macrophage phagocytosis via interaction with SIRP alpha
Abstract
Rationale: Efficient removal of apoptotic cells is essential for the resolution of acute pulmonary inflammation. Alveolar macrophages ingest apoptotic cells less avidly than other professional phagocytes at rest but overcome this defect during acute inflammation. Surfactant protein (SP)-A and SP-D are potent modulators of macrophage function and may suppress clearance of apoptotic cells through activation of the transmembrane receptor signal inhibitory regulatory protein alpha (SIRP alpha).
Objectives: To investigate whether binding of SP-A and SP-D to SIRP alpha on alveolar macrophages suppresses apoptotic cell clearance.
Methods: Phagocytosis of apoptotic cells was assessed using macrophages pretreated with SP-A, SP-D, or the collectin-like molecule C1q. Binding of SP-A and SP-D to SIRP alpha was confirmed in vitro using blocking antibodies and fibroblasts transfected with active and mutant SIRP alpha. The effects of downstream molecules SHP-1 and RhoA on phagocytosis were studied using SHP-1-deficient mice, sodium stibogluconate, and a Rho kinase inhibitor. Lipopolysaccharide was given to chimeric mice to study the effects of SP-A and SP-D binding on inflammatory macrophages.
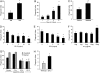
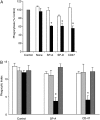
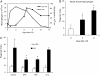
Measurements and main results: Preincubation of macrophages with SP-A or SP-D suppressed apoptotic cell clearance. Surfactant suppression of macrophage phagocytosis was reversed by blocking SIRP alpha and inhibiting downstream molecules SHP-1 and RhoA. Macrophages from inflamed lungs ingested apoptotic cells more efficiently than resting alveolar macrophages. Recruited mononuclear phagocytes with low levels of SP-A and SP-D mediated this effect.
Conclusions: SP-A and SP-D tonically inhibit alveolar macrophage phagocytosis by binding SIRP alpha. During acute pulmonary inflammation, defects in apoptotic cell clearance are overcome by recruited mononuclear phagocytes.
Figures




References
-
- Morimoto K, Amano H, Sonoda F, Baba M, Senba M, Yoshimine H, Yamamoto H, Ii T, Oishi K, Nagatake T. Alveolar macrophages that phagocytose apoptotic neutrophils produce hepatocyte growth factor during bacterial pneumonia in mice. Am J Respir Cell Mol Biol 2001;24:608–615. - PubMed
-
- Golpon HA, Fadok VA, Taraseviciene-Stewart L, Scerbavicius R, Sauer C, Welte T, Henson PM, Voelkel NF. Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J 2004;18:1716–1718. - PubMed
-
- Cox G, Crossley J, Xing Z. Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am J Respir Cell Mol Biol 1995;12:232–237. - PubMed
-
- Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am J Respir Crit Care Med 1999;160:S5–S11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

