Anticancer activity of RecQL1 helicase siRNA in mouse xenograft models
- PMID: 18422747
- PMCID: PMC11159650
- DOI: 10.1111/j.1349-7006.2008.00794.x
Anticancer activity of RecQL1 helicase siRNA in mouse xenograft models
Abstract
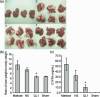
Small interfering RNAs (siRNAs) are expected to have a medical application in human therapy as drugs with a high specificity for their molecular target mRNAs. RecQL1 DNA helicase in the human RecQ helicase family participates in DNA repair and recombination pathways in the cell cycle of replication. Silencing the RecQL1 expression by RecQL1-siRNA induces mitotic death in vitro specifically in growing cancer cells. By contrast, the same RecQL1 silencing does not affect the growth of normal cells, emphasizing that RecQL1 helicase is an ideal molecular target for cancer therapy. In this study, we show that local and systemic administration of RecQL1-siRNA mixed with polyethyleneimine polymer or cationic liposomes prevented cancer cell proliferation in vivo in mouse models of cancer without noticeable adverse effects. The results indicate that RecQL1-siRNA in a complex with a cationic polymer is a very promising anticancer drug candidate, and that in particular, RecQL1-siRNA formulated with a cationic liposome has an enormous potential to be used by intravenous injection for therapy specific for liver cancers, including metastasized cancers from the colon and pancreas.
Figures





References
-
- Kawabe T, Tsuyama N, Kitao S et al . Differential regulation of human RecQ family helicases in cell transformation and cell cycle. Oncogene 2000; 19: 4764–72. - PubMed
-
- Furuichi Y. Premature aging and predisposition to cancer caused by mutations in RecQ family helicase. Ann NY Acad Sci (Rev) 2001; 928: 121–31. - PubMed
-
- Hickson DI. RecQ helicases: caretakers of the genome. Nat Rev Cancer 2003; 3: 169–78. - PubMed
-
- Shimamoto A, Sugimoto M, Furuichi Y. Molecular biology of Werner syndrome. Int J Clin Oncol 2004; 9: 288–98. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

