Early hormonal data from a multicentre phase II trial using transdermal oestrogen patches as first-line hormonal therapy in patients with locally advanced or metastatic prostate cancer
- PMID: 18422771
- PMCID: PMC2564109
- DOI: 10.1111/j.1464-410X.2008.07583.x
Early hormonal data from a multicentre phase II trial using transdermal oestrogen patches as first-line hormonal therapy in patients with locally advanced or metastatic prostate cancer
Abstract
Objective: To assess the hormonal effects of Fem7 (Merck, KGaA, Darmstadt, Germany) 100 microg transdermal oestrogen patches on men undergoing first-line androgen-deprivation therapy for prostate cancer.
Patients and methods: PATCH is a multicentre, randomized, phase II trial for men with locally advanced or metastatic prostate cancer, comparing luteinizing hormone-releasing hormone agonist therapy with oestrogen patches. To assess the dosing schedule for the patches, as this was the first time that this brand of patch had been used in men, and to reassure patients and participating clinicians, the Independent Data Monitoring Committee agreed to early release of hormonal data from this study.
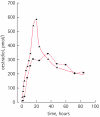
Results: Oestradiol, testosterone and prostate-specific antigen (PSA) levels are presented for the first group of 14 patients who received the patches (with 1 withdrawal) and for whom there were > or =12 weeks of follow-up by March 2007. After 12 weeks, testosterone levels (nmol/L) in eight of the 13 patients were <1.7, two were 1.7-2 and three were >2. The median (range) serum oestradiol levels was 442 (52.1-1542) pmol/L and all patients had a PSA response, with eight having a PSA level of <4 ng/mL.
Conclusion: These results confirm that oestrogen patches produce castrate levels of testosterone and concomitant PSA responses. They also highlighted the potential differences between different brands of oestrogen patches, and the need to monitor hormonal response, toxicity and efficacy until more experience with oestrogen patches for this clinical indication is obtained. The number of patches recommended in the PATCH study has now been increased.
Figures
References
-
- Carroll PR, Lee K, Fuks ZY, Kantoff PW. Cancer of the prostate. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 6th edn. Philadelphia: Lippincott, Williams and Wilkins; 2001. pp. 1435–64.
-
- Krupski TL, Smith MR, Chan Lee W, et al. Natural history of bone complications in men with prostate carcinoma initiating androgen deprivation therapy. Cancer. 2004;101:541–9. - PubMed
-
- Byar DP. Proceedings: the Veterans Administration Cooperative Urological Research Group's studies of cancer of the prostate. Cancer. 1973;32:1126–30. - PubMed
-
- Von Schoultz B, Carlstrom K, Collste L, et al. Estrogen therapy and liver function-metabolic effects of oral and parenteral administration. Prostate. 1989;14:389–95. - PubMed
-
- Ockrim JL, Lalani EN, Laniado ME, Carter SS, Abel PD. Transdermal estradiol therapy for advanced prostate cancer – forward to the past? J Urol. 2003;169:1735–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous