Basidiomycete metabolites attenuate virulence properties of Candida albicans in vitro
- PMID: 18422912
- PMCID: PMC5654498
- DOI: 10.1111/j.1439-0507.2008.01515.x
Basidiomycete metabolites attenuate virulence properties of Candida albicans in vitro
Abstract
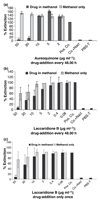
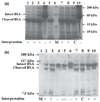
Secreted aspartic proteases (Saps) represent an important virulence factor facilitating fungal adherence. Several protease inhibitors (PIs), including HIV PIs, have been shown to reduce Candida adhesion. The aim of this study was to ascertain whether or not the recently discovered PIs Aureoquinone and Laccaridiones A and B, isolated from Basidiomycete cultures, or Bestatin, act as Sap-inhibitors and/or inhibitors of fungal adhesion. Drug effects on candidial Sap-production were determined by Sap-ELISA. Control tubes, in the absence of drug, served as positive controls, while tubes excluding both drug and proteinase induction medium were used as negative controls. Aureoquinone as well as Laccaridiones A and B, but not Bestatin, significantly inhibited Candida albicans adhesion to both epithelial and endothelial cells in a dose dependent manner and also reduced Sap-release (effects were not because of a direct interaction of the Basidiomycete metabolites with secreted Saps). Laccaridione B was consistently found to be the most effective PI tested. Interestingly, these drugs are neither fungistatic nor fungicidal at the concentrations applied. Laccaridione B may represent a promising novel type of antimycotic drug--targeting virulence factors without killing the yeast.
Figures

Similar articles
-
Tetracycline-inducible expression of individual secreted aspartic proteases in Candida albicans allows isoenzyme-specific inhibitor screening.Antimicrob Agents Chemother. 2008 Jan;52(1):146-56. doi: 10.1128/AAC.01072-07. Epub 2007 Oct 22. Antimicrob Agents Chemother. 2008. PMID: 17954688 Free PMC article.
-
Altered adherence in strains of Candida albicans harbouring null mutations in secreted aspartic proteinase genes.FEMS Microbiol Lett. 1998 Feb 1;159(1):129-35. doi: 10.1111/j.1574-6968.1998.tb12851.x. FEMS Microbiol Lett. 1998. PMID: 9485603
-
HIV protease inhibitors attenuate adherence of Candida albicans to epithelial cells in vitro.FEMS Immunol Med Microbiol. 2001 Jul;31(1):65-71. doi: 10.1111/j.1574-695X.2001.tb01588.x. FEMS Immunol Med Microbiol. 2001. PMID: 11476984
-
Aspartic protease inhibitors as potential anti-Candida albicans drugs: impacts on fungal biology, virulence and pathogenesis.Curr Med Chem. 2011;18(16):2401-19. doi: 10.2174/092986711795843182. Curr Med Chem. 2011. PMID: 21568917 Review.
-
Aspartic protease inhibitors: effective drugs against the human fungal pathogen Candida albicans.Mini Rev Med Chem. 2013 Jan;13(1):155-62. Mini Rev Med Chem. 2013. PMID: 23256485 Review.
Cited by
-
Obionin B: An o-pyranonaphthoquinone decaketide from an unidentified fungus (MSX 63619) from the Order Pleosporales.Tetrahedron Lett. 2011 Oct 5;52(40):5128-5230. doi: 10.1016/j.tetlet.2011.07.102. Tetrahedron Lett. 2011. PMID: 22025810 Free PMC article.
-
Farnesol, a fungal quorum-sensing molecule triggers Candida albicans morphological changes by downregulating the expression of different secreted aspartyl proteinase genes.Open Microbiol J. 2011;5:119-26. doi: 10.2174/1874285801105010119. Epub 2011 Nov 18. Open Microbiol J. 2011. PMID: 22207890 Free PMC article.
-
Secreted Aspartic Proteinases: Key Factors in Candida Infections and Host-Pathogen Interactions.Int J Mol Sci. 2024 Apr 27;25(9):4775. doi: 10.3390/ijms25094775. Int J Mol Sci. 2024. PMID: 38731993 Free PMC article. Review.
References
-
- Odds FC, editor. Bailliere Tindall. 2nd edition. Oxford: Elsevier; 1998. Ecology of Candida and epidemiology of candidosis; pp. 68–92.
-
- Coleman DC, Sullivan DJ, Bennett DE, Moran GP, Barry HJ, Shanley DB. Candidiasis: the emergence of a novel species, Candida dubliniensis. AIDS. 1997;11:557–67. - PubMed
-
- Odds FC, editor. Bailliere Tindall. 2nd edition. Oxford: Elsevier; 1998. Factors that predispose the host to candidosis; pp. 93–114.
-
- Davies DR. The structure and function of aspartic proteinases. Annu Rev Biophys Chem. 1990;19:189–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

