Melatonin therapy to improve nocturnal sleep in critically ill patients: encouraging results from a small randomised controlled trial
- PMID: 18423009
- PMCID: PMC2447606
- DOI: 10.1186/cc6871
Melatonin therapy to improve nocturnal sleep in critically ill patients: encouraging results from a small randomised controlled trial
Abstract
Introduction: Sleep disturbances are common in critically ill patients and when sleep does occur it traverses the day-night periods. The reduction in plasma melatonin levels and loss of circadian rhythm observed in critically ill patients receiving mechanical ventilation may contribute to this irregular sleep-wake pattern. We sought to evaluate the effect of exogenous melatonin on nocturnal sleep quantity in these patients and, furthermore, to describe the kinetics of melatonin after oral administration in this patient population, thereby guiding future dosing schedules.
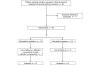
Methods: We conducted a randomised double-blind placebo-controlled trial in 24 patients who had undergone a tracheostomy to aid weaning from mechanical ventilation. Oral melatonin 10 mg or placebo was administered at 9 p.m. for four nights. Nocturnal sleep was monitored using the bispectral index (BIS) and was expressed in terms of sleep efficiency index (SEI) and area under the curve (AUC). Secondary endpoints were SEI measured by actigraphy and nurse and patient assessments. Plasma melatonin concentrations were measured in nine patients in the melatonin group on the first night.
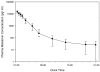
Results: Nocturnal sleep time was 2.5 hours in the placebo group (mean SEI = 0.26, 95% confidence interval [CI] 0.17 to 0.36). Melatonin use was associated with a 1-hour increase in nocturnal sleep (SEI difference = 0.12, 95% CI -0.02 to 0.27; P = 0.09) and a decrease in BIS AUC indicating 'better' sleep (AUC difference = -54.23, 95% CI -104.47 to -3.98; P = 0.04). Results from the additional sleep measurement methods were inconclusive. Melatonin appeared to be rapidly absorbed from the oral solution, producing higher plasma concentrations relative to similar doses reported in healthy individuals. Plasma concentrations declined biexponentially, but morning (8 a.m.) plasma levels remained supraphysiological.
Conclusion: In our patients, nocturnal sleep quantity was severely compromised and melatonin use was associated with increased nocturnal sleep efficiency. Although these promising findings need to be confirmed by a larger randomised clinical trial, they do suggest a possible future role for melatonin in the routine care of critically ill patients. Our pharmacokinetic analysis suggests that the 10-mg dose used in this study is too high in these patients and may lead to carryover of effects into the next morning. Reduced doses of 1 to 2 mg could be used in future studies.
Trial registration: Current Controlled Trials ISRCTN47578325.
Figures
Comment in
-
Good night, sleep tight: the time is ripe for critical care providers to wake up and focus on sleep.Crit Care. 2008;12(3):146. doi: 10.1186/cc6884. Epub 2008 May 12. Crit Care. 2008. PMID: 18492219 Free PMC article.
References
-
- American Academy of Sleep Medicine . The International Classification of Sleep Disorders: Diagnostic & Coding Manual. Westchester, IL: American Academy of Sleep Medicine; 2005.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

