The influence of venous admixture on alveolar dead space and carbon dioxide exchange in acute respiratory distress syndrome: computer modelling
- PMID: 18423016
- PMCID: PMC2447607
- DOI: 10.1186/cc6872
The influence of venous admixture on alveolar dead space and carbon dioxide exchange in acute respiratory distress syndrome: computer modelling
Abstract
Introduction: Alveolar dead space reflects phenomena that render arterial partial pressure of carbon dioxide higher than that of mixed alveolar gas, disturbing carbon dioxide exchange. Right-to-left shunt fraction (Qs/Qt) leads to an alveolar dead space fraction (VdAS/VtA; where VtA is alveolar tidal volume). In acute respiratory distress syndrome, ancillary physiological disturbances may include low cardiac output, high metabolic rate, anaemia and acid-base instability. The purpose of the present study was to analyze the extent to which shunt contributes to alveolar dead space and perturbs carbon dioxide exchange in ancillary physiological disturbances.
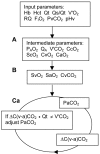
Methods: A comprehensive model of pulmonary gas exchange was based upon known equations and iterative mathematics.
Results: The alveolar dead space fraction caused by shunt increased nonlinearly with Qs/Qt and, under 'basal conditions', reached 0.21 at a Qs/Qt of 0.6. At a Qs/Qt of 0.4, reduction in cardiac output from 5 l/minute to 3 l/minute increased VdAS/VtA from 0.11 to 0.16. Metabolic acidosis further augmented the effects of shunt on VdAS/VtA, particularly with hyperventilation. A Qs/Qt of 0.5 may increase arterial carbon dioxide tension by about 15% to 30% if ventilation is not increased.
Conclusion: In acute respiratory distress syndrome, perturbation of carbon dioxide exchange caused by shunt is enhanced by ancillary disturbances such as low cardiac output, anaemia, metabolic acidosis and hyperventilation. Maintained homeostasis mitigates the effects of shunt.
Figures






Comment in
-
Causes of a high physiological dead space in critically ill patients.Crit Care. 2008;12(3):148. doi: 10.1186/cc6888. Epub 2008 May 14. Crit Care. 2008. PMID: 18492224 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

