Redox-based regulation of signal transduction: principles, pitfalls, and promises
- PMID: 18423411
- PMCID: PMC2453533
- DOI: 10.1016/j.freeradbiomed.2008.03.011
Redox-based regulation of signal transduction: principles, pitfalls, and promises
Abstract
Oxidants are produced as a by-product of aerobic metabolism, and organisms ranging from prokaryotes to mammals have evolved with an elaborate and redundant complement of antioxidant defenses to confer protection against oxidative insults. Compelling data now exist demonstrating that oxidants are used in physiological settings as signaling molecules with important regulatory functions controlling cell division, migration, contraction, and mediator production. These physiological functions are carried out in an exquisitely regulated and compartmentalized manner by mild oxidants, through subtle oxidative events that involve targeted amino acids in proteins. The precise understanding of the physiological relevance of redox signal transduction has been hampered by the lack of specificity of reagents and the need for chemical derivatization to visualize reversible oxidations. In addition, it is difficult to measure these subtle oxidation events in vivo. This article reviews some of the recent findings that illuminate the significance of redox signaling and exciting future perspectives. We also attempt to highlight some of the current pitfalls and the approaches needed to advance this important area of biochemical and biomedical research.
Figures
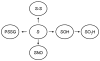




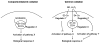
References
-
- Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. - PubMed
-
- Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. - PubMed
-
- Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. - PubMed
-
- Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE 2000. 2000:PE1. - PubMed
-
- Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01HL068865/HL/NHLBI NIH HHS/United States
- R01 HL060014/HL/NHLBI NIH HHS/United States
- P01 HL067004/HL/NHLBI NIH HHS/United States
- R01HL074295/HL/NHLBI NIH HHS/United States
- R01 HL074295/HL/NHLBI NIH HHS/United States
- R01HL60014/HL/NHLBI NIH HHS/United States
- R01HL079331/HL/NHLBI NIH HHS/United States
- R01 HL085646/HL/NHLBI NIH HHS/United States
- R01ES05511/ES/NIEHS NIH HHS/United States
- R01 HL068865/HL/NHLBI NIH HHS/United States
- P01HL67004/HL/NHLBI NIH HHS/United States
- R01 HL079331/HL/NHLBI NIH HHS/United States
- R01 ES005511/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

