Peptidomimetics, a synthetic tool of drug discovery
- PMID: 18423417
- PMCID: PMC2515564
- DOI: 10.1016/j.cbpa.2008.03.009
Peptidomimetics, a synthetic tool of drug discovery
Abstract
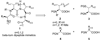
The demand for modified peptides with improved stability profiles and pharmacokinetic properties is driving extensive research effort in this field. Many structural modifications of peptides guided by rational design and molecular modeling have been established to develop novel synthetic approaches. Recent advances in the synthesis of conformationally restricted building blocks and peptide bond isosteres are discussed.
Figures
References
-
- Hruby VJ, Qiu W, Okayama T, Soloshonok VA. Design of nonpeptides from peptide ligands for peptide receptors. Methods Enzymol. 2001;343:91–123. - PubMed
-
- Cowell SM, Lee YS, Cain JP, Hruby VJ. Exploring Ramachandran and Chi space: conformationally constrained amino acids and peptides in the design of bioactive polypeptide ligands. Curr Med Chem. 2004;11:2785–2798. - PubMed
-
- Hruby VJ. Conformational restrictions of biologically active peptides via amino acid side chain groups. Life Sci. 1982;31:189–199. - PubMed
-
- Kessler H. Peptide conformations. Part 19. Conformation and biological effects of cyclic peptides. Angewandte Chem Int Ed Engl. 1982;94:509–520.
-
- Lam KS, Salmon SE, Hersh EM, Hruby VJ, Kazmierski WM, Knapp RJ. A new type of synthetic peptide library for identifying ligand-binding activity. Nature (London, United Kingdom) 1991;354:82–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources