Nuclear trafficking of the human cytomegalovirus pp71 (ppUL82) tegument protein
- PMID: 18423509
- PMCID: PMC2464290
- DOI: 10.1016/j.virol.2008.03.007
Nuclear trafficking of the human cytomegalovirus pp71 (ppUL82) tegument protein
Abstract
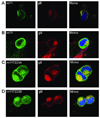
The human cytomegalovirus tegument protein pp71 localizes to the nucleus immediately upon infection, and functions to initiate viral gene expression. Analysis of a series of random insertion mutations revealed that sequences within the mid region (MR) of pp71 are important for localization to the nucleus. Fusion of MR sequences with eGFP revealed that amino acids 94 to 300 were sufficient to target proteins to the nucleus. Random substitution mutagenesis within this domain resulted in two double substitution mutants, pp71P203T/T223M and pp71T228M/L275Q, with a predominantly cytoplasmic localization. Disruption of nuclear targeting resulted in relocalization of the fusion proteins to a distinct perinuclear region. Using tandem mass spectrometry, we determined that threonine 223 can be phosphorylated. Mutation of this residue to a phosphomimetic amino acid resulted in abrogation of nuclear targeting. These results strongly suggest that the intracellular trafficking of pp71 is regulated by phosphorylation.
Figures


References
-
- Anders DG, Kerry JA, Pari GS. Betaherpesviruses: DNA synthesis and late viral gene expression. In: Arvin A, Campadielli-Fume G, Mocarski ES, Moore P, Roizman B, Whitley R, Yamanishi K, editors. "Human Herpesviruses: Biology, Therapy and Immunoprophylaxis". Cambridge: Cambridge University Press; 2007. pp. 295–310.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

