Mutation in the human homeobox gene NKX5-3 causes an oculo-auricular syndrome
- PMID: 18423520
- PMCID: PMC2427260
- DOI: 10.1016/j.ajhg.2008.03.007
Mutation in the human homeobox gene NKX5-3 causes an oculo-auricular syndrome
Abstract
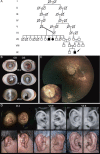
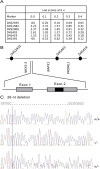
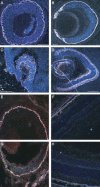
Several dysmorphic syndromes affect the development of both the eye and the ear, but only a few are restricted to the eye and the external ear. We describe a developmental defect affecting the eye and the external ear in three members of a consanguineous family. This syndrome is characterized by ophthalmic anomalies (microcornea, microphthalmia, anterior-segment dysgenesis, cataract, coloboma of various parts of the eye, abnormalities of the retinal pigment epithelium, and rod-cone dystrophy) and a particular cleft ear lobule. Linkage analysis and mutation screening revealed in the first exon of the NKX5-3 gene a homozygous 26 nucleotide deletion, generating a truncating protein that lacked the complete homeodomain. Morpholino knockdown expression of the zebrafish nkx5-3 induced microphthalmia and disorganization of the developing retina, thus confirming that this gene represents an additional member implicated in axial patterning of the retina.
Figures



References
-
- Hever A.M., Williamson K.A., van Heyningen V. Developmental malformations of the eye: The role of PAX6, SOX2 and OTX2. Clin. Genet. 2006;69:459–470. - PubMed
-
- Represa J., Frenz D.A., Van De Water T.R. Genetic patterning of embryonic inner ear development. Acta Otolaryngol. 2000;120:5–10. - PubMed
-
- Franceschetti A., Valerio M. Confin. Neurol. 1945;6:255–257. - PubMed
-
- Stadler H.S., Murray J.C., Leysens N.J., Goodfellow P.J., Solursh M. Phylogenetic conservation and physical mapping of members of the H6 homeobox gene family. Mamm. Genome. 1995;6:383–388. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

