In the ventral cochlear nucleus Kv1.1 and subunits of HCN1 are colocalized at surfaces of neurons that have low-voltage-activated and hyperpolarization-activated conductances
- PMID: 18424000
- PMCID: PMC2493296
- DOI: 10.1016/j.neuroscience.2008.01.085
In the ventral cochlear nucleus Kv1.1 and subunits of HCN1 are colocalized at surfaces of neurons that have low-voltage-activated and hyperpolarization-activated conductances
Abstract
Principal cells of the ventral cochlear nucleus (VCN) differ in the magnitudes of low-voltage-activated potassium (gKL) and hyperpolarization-activated (gh) conductances that determine the time course of signaling. Octopus cells in mice have large gKL (500 nS) and gh (150 nS), bushy cells have smaller gKL (80 nS) and gh (30 nS), and T stellate cells have little gKL and a small gh (20 nS). gKL Arises through potassium channels of which approximately 60% contain Kv1.1 (potassium channels in the shaker or KCNA family) subunits; gh arises through channels that include hyperpolarization and cyclic nucleotide gated (HCN) 1 subunits. The surfaces of cell bodies and dendrites of octopus cells in the dorsocaudal pole, and of similar cells along the ventrolateral edge of the PVCN, were brightly labeled by an antibody against HCN1 that was colocalized with labeling for Kv1.1. More anteriorly neurons with little surface labeling were intermingled among cell bodies and dendrites with surface labeling for both proteins, likely corresponding to T stellate and bushy cells. The membrane-associated labeling patterns for Kv1.1 and HCN1 were consistent with what is known about the distribution and the electrophysiological properties of the principal cells of the VCN. The cytoplasm of large cells and axonal paranodes contained immunofluorescent labeling for only Kv1.1.
Figures

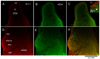
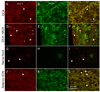

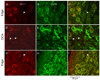
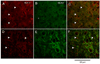
References
-
- Adamson CL, Reid MA, Mo ZL, Bowne-English J, Davis RL. Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J Comp Neurol. 2002;447:331–350. - PubMed
-
- Bal R, Oertel D. Hyperpolarization-activated, mixed-cation current (Ih) in octopus cells of the mammalian cochlear nucleus. J Neurophysiol. 2000;84:806–817. - PubMed
-
- Bal R, Oertel D. Potassium currents in octopus cells of the mammalian cochlear nuclei. J Neurophysiol. 2001;86:2299–2311. - PubMed
-
- Barnes-Davies M, Barker MC, Osmani F, Forsythe ID. Kv1 currents mediate a gradient of principal neuron excitability across the tonotopic axis in the rat lateral superior olive. Eur J Neurosci. 2004;19:325–333. - PubMed
-
- Bekele-Arcuri Z, Matos MF, Manganas L, Strassle BW, Monaghan MM, Rhodes KJ, Trimmer JS. Generation and characterization of subtype-specific monoclonal antibodies to K+ channel alpha- and beta-subunit polypeptides. Neuropharm. 1996;35:851–865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

