Functional analysis of the protein machinery required for transport of lipopolysaccharide to the outer membrane of Escherichia coli
- PMID: 18424520
- PMCID: PMC2446812
- DOI: 10.1128/JB.00270-08
Functional analysis of the protein machinery required for transport of lipopolysaccharide to the outer membrane of Escherichia coli
Abstract
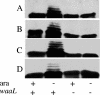
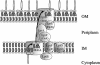
Lipopolysaccharide (LPS) is an essential component of the outer membrane (OM) in most gram-negative bacteria, and its structure and biosynthetic pathway are well known. Nevertheless, the mechanisms of transport and assembly of this molecule at the cell surface are poorly understood. The inner membrane (IM) transport protein MsbA is responsible for flipping LPS across the IM. Additional components of the LPS transport machinery downstream of MsbA have been identified, including the OM protein complex LptD/LptE (formerly Imp/RlpB), the periplasmic LptA protein, the IM-associated cytoplasmic ATP binding cassette protein LptB, and LptC (formerly YrbK), an essential IM component of the LPS transport machinery characterized in this work. Here we show that depletion of any of the proteins mentioned above leads to common phenotypes, including (i) the presence of abnormal membrane structures in the periplasm, (ii) accumulation of de novo-synthesized LPS in two membrane fractions with lower density than the OM, and (iii) accumulation of a modified LPS, which is ligated to repeating units of colanic acid in the outer leaflet of the IM. Our results suggest that LptA, LptB, LptC, LptD, and LptE operate in the LPS assembly pathway and, together with other as-yet-unidentified components, could be part of a complex devoted to the transport of LPS from the periplasmic surface of the IM to the OM. Moreover, the location of at least one of these five proteins in every cellular compartment suggests a model for how the LPS assembly pathway is organized and ordered in space.
Figures




References
-
- Bachmann, B. J. 1987. Derivatives and genotypes of some mutant derivatives of Escherichia coli K-12, p. 1191-1219. In J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. ASM Press, Washington, DC.
-
- Bayer, M. E. 1968. Areas of adhesion between wall and membrane of Escherichia coli. J. Gen. Microbiol. 53395-404. - PubMed
-
- Bayer, M. E. 1991. Zones of membrane adhesion in the cryofixed envelope of Escherichia coli. J. Struct. Biol. 107268-280. - PubMed
-
- Bos, M. P., V. Robert, and J. Tommassen. 2007. Biogenesis of the gram-negative bacterial outer membrane. Annu. Rev. Microbiol. 61191-214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

