The cyclic AMP-dependent catabolite repression system of Serratia marcescens mediates biofilm formation through regulation of type 1 fimbriae
- PMID: 18424546
- PMCID: PMC2423026
- DOI: 10.1128/AEM.02733-07
The cyclic AMP-dependent catabolite repression system of Serratia marcescens mediates biofilm formation through regulation of type 1 fimbriae
Abstract
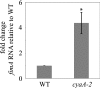
The mechanisms by which environmental carbon sources regulate biofilm formation are poorly understood. This study investigates the roles of glucose and the catabolite repression system in Serratia marcescens biofilm formation. The abilities of this opportunistic pathogen to proliferate in a wide range of environments, to cause disease, and to resist antimicrobials are linked to its ability to form biofilms. We observed that growth of S. marcescens in glucose-rich medium strongly stimulated biofilm formation, which contrasts with previous studies showing that biofilm formation is inhibited by glucose in Escherichia coli and other enteric bacteria. Glucose uptake is known to inversely mediate intracellular cyclic AMP (cAMP) synthesis through regulation of adenylate cyclase (cyaA) activity, which in turn controls fundamental processes such as motility, carbon utilization and storage, pathogenesis, and cell division in many bacteria. Here, we demonstrate that mutation of catabolite repression genes that regulate cAMP levels (crr and cyaA) or the ability to respond to cAMP (crp) confers a large increase in biofilm formation. Suppressor analysis revealed that phenotypes of a cAMP receptor protein (crp) mutant require the fimABCD operon, which is responsible for type 1 fimbria production. Consistently, fimA transcription and fimbria production were determined to be upregulated in a cyaA mutant background by using quantitative real-time reverse transcription-PCR and transmission electron microscopy analysis. The regulatory pathway by which environmental carbon sources influence cAMP concentrations to alter production of type 1 fimbrial adhesins establishes a novel mechanism by which bacteria control biofilm development.
Figures



References
-
- Adamo, S. A. 2004. Estimating disease resistance in insects: phenoloxidase and lysozyme-like activity and disease resistance in the cricket Gryllus texensis. J. Insect Physiol. 50:209-216. - PubMed
-
- Barnich, N., J. Boudeau, L. Claret, and A. Darfeuille-Michaud. 2003. Regulatory and functional co-operation of flagella and type 1 pili in adhesive and invasive abilities of AIEC strain LF82 isolated from a patient with Crohn's disease. Mol. Microbiol. 48:781-794. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

