Cutting edge: Foxp3-mediated induction of pim 2 allows human T regulatory cells to preferentially expand in rapamycin
- PMID: 18424697
- PMCID: PMC2504517
- DOI: 10.4049/jimmunol.180.9.5794
Cutting edge: Foxp3-mediated induction of pim 2 allows human T regulatory cells to preferentially expand in rapamycin
Abstract
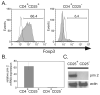
Addition of rapamycin to cultures of expanding natural CD4+CD25+Foxp3+ T regulatory cells (Tregs) helps maintain their suppressive activity, but the underlying mechanism is unclear. Pim 2 is a serine/threonine kinase that can confer rapamycin resistance. Unexpectedly, pim 2 was found to be constitutively expressed in freshly isolated, resting Tregs, but not in CD4+CD25- T effector cells. Introduction of Foxp3, but not Foxp3Delta2, into effector T cells induced pim 2 expression and conferred preferential expansion in the presence of rapamycin, indicating that Foxp3 can regulate pim 2 expression. Finally, we determined there is a positive correlation between Treg expansion and Foxp3 expression in the presence of rapamycin. Together, these results indicate that Tregs are programmed to be resistant to rapamycin, providing further rationale for why this immunosuppressive drug should be used in conjunction with expanded Tregs.
Figures


References
-
- Bluestone JA. Regulatory T-cell therapy: is it ready for the clinic? Nat Rev Immunol. 2005;5:343–349. - PubMed
-
- Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. - PubMed
-
- Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. - PubMed
-
- Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–8347. - PubMed
-
- Coenen JJA, Koenen HJPM, van Rijssen E, Hilbrands LB, Joosten I. Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood. 2006;107:1018–1023. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

