Bone morphogenetic protein 2 mediates dentin sialophosphoprotein expression and odontoblast differentiation via NF-Y signaling
- PMID: 18424784
- PMCID: PMC2443643
- DOI: 10.1074/jbc.M709492200
Bone morphogenetic protein 2 mediates dentin sialophosphoprotein expression and odontoblast differentiation via NF-Y signaling
Abstract
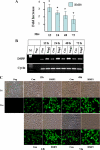
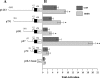
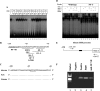
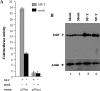
Dentin sialophosphoprotein (DSPP), an important odontoblast differentiation marker, is necessary for tooth development and mineralization. Bone morphogenetic protein 2 (BMP2) plays a vital role in odontoblast function via diverse signal transduction systems. We hypothesize that BMP2 regulates DSPP gene transcription and thus odontoblast differentiation. Here we report that expression of BMP2 and DSPP is detected during mouse odontogenesis by in situ hybridization assay, and BMP2 up-regulates DSPP mRNA and protein expression as well as DSPP-luciferase promoter activity in mouse preodontoblasts. By sequentially deleting fragments of the mouse DSPP promoter, we show that a BMP2-response element is located between nucleotides -97 and -72. By using antibody and oligonucleotide competition assays in electrophoretic mobility shift analysis and chromatin immunoprecipitation experiments, we show that the heterotrimeric transcription factor Y (NF-Y) complex physically interacts with the inverted CCAAT box within the BMP2-response element. BMP2 induces NF-Y accumulation into the nucleus increasing its recruitment to the mouse DSPP promoter in vivo. Furthermore, forced overexpression of NF-Y enhances promoter activity and increases endogenous DSPP protein levels. In contrast, mutations in the NF-Y-binding motif reduce BMP2-induced DSPP transcription. Moreover, inhibiting BMP2 signaling by Noggin, a BMP2 antagonist, results in significant inhibition of DSPP gene expression in preodontoblasts. Taken together, these results indicate that BMP2 mediates DSPP gene expression and odontoblast differentiation via NF-Y signaling during tooth development.
Figures




References
-
- Thesleff, I. (2003) J. Cell Sci. 116 1647–1648 - PubMed
-
- Maas, R., and Bei, M. (1997) Crit. Rev. Oral Biol. Med. 8 4–39 - PubMed
-
- Miletich, I., and Sharpe, P. T. (2003) Hum. Mol. Genet. 1 R69–R73 - PubMed
-
- D'Souza, R. N., Cavender, A., Sunavala, G., Alvarez, J., Ohshima, T., Kulkarni, A. B., and MacDougall, M. (1997) J. Bone Miner. Res. 12 2040–2049 - PubMed
-
- Chen, S., Rani, S., Wu, Y., Unterbrink, A., Gu, T. T., Gluhak-Heinrich, J., Chuang, H. H., and MacDougall, M. (2005) J. Biol. Chem. 280 29717–29727 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

