Quantal noise from human red cone pigment
- PMID: 18425122
- PMCID: PMC2774794
- DOI: 10.1038/nn.2110
Quantal noise from human red cone pigment
Abstract
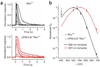
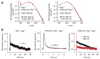
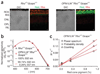
The rod pigment, rhodopsin, shows spontaneous isomerization activity. This quantal noise produces a dark light of approximately 0.01 photons s(-1) rod(-1) in human, setting the threshold for rod vision. The spontaneous isomerization activity of human cone pigments has long remained a mystery because the effect of a single isomerized pigment molecule in cones, unlike that in rods, is small and beyond measurement. We have now overcome this problem by expressing human red cone pigment transgenically in mouse rods in order to exploit their large single-photon response, especially after genetic removal of a key negative-feedback regulation. Extrapolating the measured quantal noise of transgenic cone pigment to native human red cones, we obtained a dark rate of approximately 10 false events s(-1) cone(-1), almost 10(3)-fold lower than the overall dark transduction noise previously reported in primate cones. Our measurements provide a rationale for why mammalian red, green and blue cones have comparable sensitivities, unlike their amphibian counterparts.
Figures

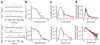

References
-
- Barlow HB. Visual Problems of Colour. Vol. II. London: Her Majesty’s Stationery Office; 1958. Intrinsic noise of cones; pp. 617–630. (ed. National Physical Laboratory, Teddington, England)
-
- Donner K. Noise and the absolute thresholds of cone and rod vision. Vision Res. 1992;32:853–866. - PubMed
-
- Field GD, Sampath AP, Rieke F. Retinal processing near absolute threshold: from behaviour to mechanism. Annu. Rev. Physiol. 2005;67:491–514. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

