Ciclesonide versus placebo for chronic asthma in adults and children
- PMID: 18425941
- PMCID: PMC7387112
- DOI: 10.1002/14651858.CD006217.pub2
Ciclesonide versus placebo for chronic asthma in adults and children
Abstract
Background: Inhaled corticosteroids are an integral part of asthma management, and act as an anti-inflammatory agent in the airways of the lung. These agents confer significant benefit in terms of symptom management and improvement in lung function, but may also cause harm in terms of local and systemic side-effects. Ciclesonide is a novel steroid that has efficient distribution and release properties that mean it can be taken once daily, making it potentially useful in ongoing asthma management.
Objectives: To assess the efficacy of inhaled ciclesonide in adults and children with chronic asthma.
Search strategy: We searched the Cochrane Airways Group register of trials with pre-defined terms. Additional searches of CENTRAL and PubMed were undertaken. The literature searches for this review are current up to June 2007.
Selection criteria: Randomised parallel or crossover studies were eligible for the review. We included studies comparing ciclesonide with placebo, and we also included studies comparing ciclesonide at different doses.
Data collection and analysis: Two authors assessed studies for inclusion in the review, extracted data independently and checked each others' work. We contacted study investigators in order to obtain additional data. Extracted data were entered into RevMan 4.2 and analysed as fixed effect mean differences for continuous data, and fixed effect risk ratios for dichotomous data.
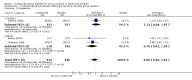
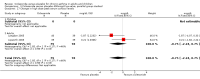
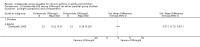
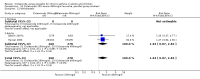
Main results: Eighteen trials (reporting 20 study comparisons) met the review entry criteria. We report findings from 18 group comparisons where data were available (6343 participants, of whom 1692 were children). Ciclesonide versus placebo: The short duration of the included studies means that there is a lack of data with respect to the impact of ciclesonide on asthma exacerbations. At doses of 100 mcg/d or less up to 400 mcg/d in mild to moderate asthma, ciclesonide improved lung function, asthma symptoms and rescue inhaler use, compared with placebo.Dose response outcomes: Comparisons of 100 versus 200 mcg/d, 100 versus 400 mcg/d and 400 versus 800 mcg/d did not yield significant differences in lung function outcomes. Adverse event data were not available in sufficient detail to permit assessment of the safety profile of this drug.
Authors' conclusions: Ciclesonide was more effective than placebo, in the short term, in improving lung function in patients with mild to moderate asthma previously treated with inhaled corticosteroids. There remain questions as to dose response, and the lack of data on the longer term impact on exacerbations and safety profile should be addressed in future studies.
Conflict of interest statement
None known.
Figures



















































































































































































































Update of
- doi: 10.1002/14651858.CD006217
Similar articles
-
Ciclesonide versus other inhaled steroids for chronic asthma in children and adults.Cochrane Database Syst Rev. 2008 Apr 16;2008(2):CD007031. doi: 10.1002/14651858.CD007031. Cochrane Database Syst Rev. 2008. PMID: 18425977 Free PMC article.
-
Ciclesonide versus other inhaled corticosteroids for chronic asthma in children.Cochrane Database Syst Rev. 2013 Feb 28;2013(2):CD010352. doi: 10.1002/14651858.CD010352. Cochrane Database Syst Rev. 2013. PMID: 23450613 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Interventions to improve inhaler technique for people with asthma.Cochrane Database Syst Rev. 2017 Mar 13;3(3):CD012286. doi: 10.1002/14651858.CD012286.pub2. Cochrane Database Syst Rev. 2017. PMID: 28288272 Free PMC article.
-
Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD007524. doi: 10.1002/14651858.CD007524.pub5. Cochrane Database Syst Rev. 2022. PMID: 36161875 Free PMC article.
Cited by
-
Ciclesonide versus other inhaled steroids for chronic asthma in children and adults.Cochrane Database Syst Rev. 2008 Apr 16;2008(2):CD007031. doi: 10.1002/14651858.CD007031. Cochrane Database Syst Rev. 2008. PMID: 18425977 Free PMC article.
-
Guidelines for diagnosis and management of bronchial asthma: Joint ICS/NCCP (I) recommendations.Lung India. 2015 Apr;32(Suppl 1):S3-S42. doi: 10.4103/0970-2113.154517. Lung India. 2015. PMID: 25948889 Free PMC article. No abstract available.
-
Addition of long-acting beta2-agonists to inhaled corticosteroids for chronic asthma in children.Cochrane Database Syst Rev. 2015 Nov 24;2015(11):CD007949. doi: 10.1002/14651858.CD007949.pub2. Cochrane Database Syst Rev. 2015. PMID: 26594816 Free PMC article.
-
Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children.Cochrane Database Syst Rev. 2010 May 12;2010(5):CD005535. doi: 10.1002/14651858.CD005535.pub2. Cochrane Database Syst Rev. 2010. PMID: 20464739 Free PMC article.
-
Large care gaps in primary care management of asthma: a longitudinal practice audit.BMJ Open. 2019 Jan 29;9(1):e022506. doi: 10.1136/bmjopen-2018-022506. BMJ Open. 2019. PMID: 30696669 Free PMC article.
References
References to studies included in this review
Adachi 2007a {published data only}
-
- Adachi M, Ishihara K, Inoue H, Kudo K, Takahashi K, Morita Y, et al. Efficacy and safety of once‐daily inhaled ciclesonide in adults with mild to moderate asthma: A double‐blind, placebo‐controlled study. Respirology 2007;12(4):566‐72. - PubMed
Adachi 2007b {published data only}
-
- Adachi M, Ishihara K, Inoue H, Kudo K, Takahashi K, Morita Y, et al. Efficacy and safety of inhaled ciclesonide compared with chlorofluorocarbon beclomethasone dipropionate in adults with moderate to severe persistent asthma. Respirology 2007;12(4):573‐80. - PubMed
Baena Cagnani 2006 {unpublished data only}
-
- Baena‐Cagnani CE, Mansfeild L, Zhang P, Lloyd M, Banjeri D. Once daily ciclesonide has no effect on growth in children with mild persistent asthma a subgroup analysis of north and South American populations. Journal of Allergy and Clinical Immunology 2006;117(2 Suppl 1):S191.
-
- Bernstein D, Caballero‐Fonseca F, Zhang P, Lloyd M, Banjeri D. Once daily ciclesonide has no effect on hypothalmic pituitary adrenal axis function in prepubertal children with mild persistent asthma. Journal of Allergy and Clinical Immunology 2006;117(2 Suppl 1):S184.
-
- Maspero J, Maika S, Kundu S, Lloyd M, Banjerji D. Ciclesonide once daily is well tolerated in children with mild persistent asthma a 1 year double blind placebo controlled study. Journal of Allergy and Clinical Immunology 2006;117(2 Suppl 1):S94.
-
- Nayak A, Herrera O, Kudu S, Lloyd M, Banjeri D. Ciclesonide has no effect on growth in prepubertal children with mild persistent asthma irrespective of age and gender. Journal of Allergy and Clinical Immunology 2006;117(2 Suppl 1):S191.
-
- Neffen H, Ruff M, Zhang P, Lloyd M, Banjeri D. Ciclesonide administered once daily has no effect on skeletal maturity in prepubertal children with mild persistent asthma. Journal of Allergy and Clinical Immunology 2006;117(2 Suppl 1):S184.
Bateman 2006 {published data only}
-
- Bateman E, Karpel J, Casale T, Craig T, Wenzel S, Fish J, et al. Ciclesonide reduces oral corticosteroid use in adults with severe persistent asthma while maintaining asthma control. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B35; Poster: G16.
-
- Bateman E, Karpel J, Casale T, Wenzel S, Banerji D. Ciclesonide reduces the need for oral steroid use in adult patients with severe, persistent asthma. Chest 2006;129(5):1176‐87. - PubMed
Bateman 2006a {published data only}
-
- Bateman ED, Velazquez‐Samano G, Záková L, Chuchalin A, Göhring UM, Engelstätter R. Comparison of ciclesonide 160 versus 640 mcg per day in patients with severe asthma. European Respiratory Journal 2006;28(50):206s.
Bernstein 2004 {unpublished data only}
-
- Bernstein D, Berger W, Welch M, Kundu S, Banerji D. Once‐daily ciclesonide significantly improves pulmonary function in adults/adolescents with mild‐moderate asthma. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B35; poster: G27.
-
- Bernstein D, Nathan R, Ledford D, Ledoux E, Pedinoff A, Crivera C, et al. Ciclesonide, a new inhaled corticosteroid, significantly improves asthma‐related quality of life in patients with severe, persistent asthma. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S210.
-
- Bernstein JA, Noonan MJ, Rim C, Fish J, Kundu S, Williams J, et al. Ciclesonide has minimal oropharyngeal side effects in the treatment of patients with moderate‐to‐severe asthma. Journal of Allergy and Clinical Immunology 2004;113(2 Suppl):S113.
-
- Busse W, Kaliner M, Bernstein D, Nayak A, Kundu S, Williams J. The novel inhaled corticosteroid ciclesonide is efficacious and has a favourable safety profile in adults and adolescents with severe persistent asthma. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S213.
-
- Skoner D, Baena‐Cagnani CE, Maspero J, Neffen H, Banerji D. Once‐daily ciclesonide has no effect on growth velocity, as assessed by stadiometer height, in children with mild, persistent asthma. American Thoracic Society International Conference, San Diego, California, May 19‐24, 2006. 2006:A73 [Poster G20].
Chapman 2005 {published data only}
-
- Chapman KR, Buhl R, Engelstätter R. Once daily ciclesonide is effective in the treatment of asthma. Thorax 2004;59(Suppl 2):ii71.
-
- Chapman KR, D'Urzo AD, Oedekoven C, Steinijans VW, Wurst W. Effects of ciclesonide versus placebo on lung function after 12 weeks of treatment in patients with asthma. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A767.
-
- Chapman KR, Patel P, Boulet LP, D'Urzo AD, Alexander M, Mehra S, et al. Efficacy and long‐term safety of ciclesonide in asthmatic patients as demonstrated in a 52 week long study [abstract]. European Respiratory Society Annual Congress. 2002:Abstract nr: 2328.
-
- Chapman KR, Patel P, D'Urzo AD, Alexander M, Mehra S, Oedekoven C, et al. Maintenance of asthma control by once‐daily inhaled ciclesonide in adults with persistent asthma. Allergy 2005;60(3):330‐7. - PubMed
DFI6153 {unpublished data only}
-
- Sanofi‐Aventis. A placebo‐ and active‐controlled (ciclesonide metered‐dose inhaler), randomized, parallel‐group, dose‐range finding study of ciclesonide administered by dry powder inhaler (Ultrahaler®) in adult and adolescent patients with persistent asthma. http://www.clinicalstudyresults.org [Accessed June 2007] 2006.
EFC6163a {published data only}
-
- Sanofi‐Aventis. A multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study to assess the efficacy of ciclesonide metered‐dose inhaler at a daily dose of 160 mcg administered for 12 weeks either in a once‐daily regimen in the morning (160 mcg qd AM) or in a twice‐daily regimen (80 mcg bid) in adults and adolescents with mild to moderate persistent asthma treated previously with inhaled corticosteroids. http://www.clinicalstudyresults.org [Accessed June 2007] 2006.
EFC6163b {unpublished data only}
-
- Sanofi‐Aventis. A multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study to assess the efficacy of ciclesonide metered‐dose inhaler at a daily dose of 160 mcg administered for 12 weeks either in a once‐daily regimen in the morning (160 mcg qd AM) or in a twice‐daily regimen (80 mcg bid) in adults and adolescents with mild to moderate persistent asthma treated previously with inhaled corticosteroids. http://www.clinicalstudyresults.org [Accessed June 2007] 2006.
Gelfand 2006a {published data only}
-
- Gelfand E, Boguniweicz M, Weinstein S, Bernstein C, Lloyd M, Zhang P, et al. Ciclesonide, administered once daily, has a low incidence of oropharyngeal adverse events in pediatric asthma patients. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S213.
-
- Gelfand EW, Georgitis JW, Noonan M, Ruff ME. Once‐daily ciclesonide in children: efficacy and safety in asthma. Journal of Pediatrics 2006;148(3):377‐83. - PubMed
-
- Kerwin E, Meltzer E, Kundo S, Banjeri D. Ciclesonide an effective once‐daily inhaled corticosteroid for children with persistent asthma. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:C47; Poster: A11.
-
- Miller D, Ratner P, Condemi J, Lawrence M, Crivera C, Lloyd M, et al. Once‐daily ciclesonide improves quality of life in pediatric patients with asthma. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S211.
Gelfand 2006b {published data only}
-
- Gelfand E, Boguniweicz M, Weinstein S, Bernstein C, Lloyd M, Zhang P, et al. Ciclesonide, administered once daily, has a low incidence of oropharyngeal adverse events in pediatric asthma patients. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S213.
-
- Gelfand EW, Georgitis JW, Noonan M, Ruff ME. Once‐daily ciclesonide in children: efficacy and safety in asthma. Journal of Pediatrics 2006;148(3):377‐83. - PubMed
-
- Kerwin E, Meltzer E, Kundo S, Banjeri D. Ciclesonide an effective once‐daily inhaled corticosteroid for children with persistent asthma. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:C47; Poster: A11.
-
- Miller D, Ratner P, Condemi J, Lawrence M, Crivera C, Lloyd M, et al. Once‐daily ciclesonide improves quality of life in pediatric patients with asthma. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S211.
Hansel 2006 {published data only}
-
- Engelstätter R, Benezet O, Kafe H, Ponitz HH, Hansel T, Cheung D, et al. Comparative study in asthma patients treated with inhaled ciclesonide (80µg or 320µg once daily) or budesonide (200µg twice daily) for 12 weeks. American Thoracic Society 99th International Conference. 2003; Vol. C105:D64.
-
- Hansel T, Biberger C, Engelstätter R. Ciclesonide 80 µg or 320 µg once daily achieves lung function improvement comparable with budesonide 200 µg twice daily in patients with persistent asthma. Thorax 2004;59(Suppl II):ii71.
-
- Hansel T, Engelstatter R, Benezet O, Kafe H, Ponitz HH, Cheung D, et al. Once daily ciclesonide (80ug or 320ug) is equally effective as budesonide 200ug given twice daily: a 12 week study in asthma patients. European Respiratory Journal 2003;22(Suppl 45):Abstract No: P2639.
-
- Hansel TT, Benezet O, Kafe H, Ponitz HH, Cheung D, Engelstatter R, Barnes PJ. A multinational, 12‐week, randomized study comparing the efficacy and tolerability of ciclesonide and budesonide in patients with asthma. Clinical therapeutics 2006;28(6):906‐20. - PubMed
Langdon 2005 {published data only}
-
- Drollmann A, Langdon C, Engelstätter R, Rathgeb F, Steinijans VW, Wurst W. Ciclesonide is effective in the treatment of bronchial asthma. European Respiratory Journal 2001;18(Suppl 33):95s.
-
- Engelstätter R, Langdon C, Bethke T, Rathgeb F, Steinijans VW, Wurst W. Efficacy of ciclesonide after 12 week treatment of bronchial asthma. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A766.
-
- Langdon CG, Adler M, Mehra S, Alexander M, Drollmann A. Once‐daily ciclesonide 80 or 320mug for 12 weeks is safe and effective in patients with persistent asthma. Respiratory Medicine 2005;99(10):1275‐85. - PubMed
Lipworth 2005 {published data only}
-
- LaForce CF, Baker JW, Amin D, Rohatagi S, Mendes P, Williams J, et al. Ciclesonide a novel inhaled steroid has no effect on hypothalamic pituitary adrenal (HPA) ‐ axis function in mild to moderate asthmatics. Journal of Allergy and Clinical Immunology 2003;111(2 Suppl):S218.
-
- Lipworth BJ, Kaliner MA, LaForce CF, Baker JW, Kaiser HB, Amin D, et al. Effect of ciclesonide and fluticasone on hypothalamic‐pituitary‐adrenal axis function in adults with mild‐to‐moderate persistent asthma. Annals of Allergy, Asthma and Immunology 2005;94(4):465‐72. - PubMed
Magnussen 2007 {published data only}
-
- Magnussen H, Hellwig M, Hellbradt S, Josten JP, Engelstätter R. Ciclesonide 80ug or 160ug once‐daily and fluticasone propionate 88ug twice‐daily similarly reduce asthma symptoms and rescue medication use in patients with mild‐to‐moderate asthma. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B35; Poster: G20.
-
- Magnussen H, Hofman J, Novakova B, Kaczmarek B, Hellwig M, Engelstätter R. Ciclesonide 80 µg or 160 µg once daily is comparable to fluticasone propionate 88 µg twice daily in the treatment of asthma patients. European Respiratory Journal 2005;26(Suppl 49):255s.
-
- Magnussen H, Hofman J, Novakova B, Kaczmarek B, Hellwig M, Engelstätter R. Ciclesonide decreases exhaled breath nitric oxide and rapidly reduces airway responsiveness to adenosine monophosphate in asthma patients. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1723.
-
- Magnussen H, Hofman J, Novakova B, Kaczmarek‐Czeczothka B, Koci T, Staneta P, et al. Ciclesonide 80 µg or 160 µg once‐daily is as effective as fluticasone propionate 88 µg twice‐daily in the treatment of persistent asthma. Journal of Allergy and Clinical Immunology 2005;115(2 Suppl):S4.
-
- Magnussen H, Hofman J, Novokova B, Kaczmarek B, Schutze J, Hellwig M. Once daily ciclesonide 80 µg or 160 µg is comparable to twice daily fluticasone propionate 88 µg in the treatment of persistent asthma. XIX World Allergy Organization Congress; Munich, Germany. 2005:Abstract 297.
O'Connor 2002 {published data only}
-
- O'Connor B, Sips P, Engelstätter R, Steinijans VW, Wurst W. Management of moderate to severe bronchial asthma by ciclesonide: A 12‐week trial. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A767.
-
- O'Connor BJ, Kilfeather S, Cheung D, Sips P, Blagden MD, Kafe H, et al. Treatment of moderate to severe asthma with ciclesonide: a long‐term investigation over 52 weeks. European Respiratory Society Annual Congress 2002. 2002:Abstract nr: 2579.
Pearlman 2005 {published data only}
-
- Berger WE. Ciclesonide is well tolerated and has minimal oropharyngeal side effects at once‐daily doses of 80µg, 160µg and 320µg in the treatment of patients with mild to moderate asthma. Journal of Allergy and Clinical Immunology 2004;113(2 Suppl):S38.
-
- Bernstein D, Berger W, Welch M, Kundu S, Banerji D. Once‐daily ciclesonide significantly improves pulmonary function in adults/adolescents with mild‐moderate asthma. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B35; poster: G27.
-
- Kerwin E, Chervinsky P, Fish J, Kundu S, Williams J, Banerji D, et al. Ciclesonide has no effect on hypothalamic‐pituitary‐adrenal (HPA) axis at once‐daily doses of 80 mcg, 160 mcg, or 320 mcg in the treatment of patients with mild‐to‐moderate asthma. American Thoracic Society 100th International Conference, May 21‐26, 2004, Orlando. 2004:A 37; Poster J104.
-
- Nayak A, Charous BL, Finn A, Lumry W, Crivera C, Williams J, et al. A novel inhaled corticosteroid ciclesonide significantly improves quality of life in patients with mild‐to‐moderate asthma. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S210.
-
- Pearlman D, Creticos P, Lampl K, Gower R, Kundu S, Fish J, et al. Once‐daily ciclesonide is effective and well‐tolerated in adult and adolescent patients with mild‐to‐moderate asthma. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S212. - PubMed
Wilson 2006 {published data only}
-
- Wilson AM, Duong M, Pratt B, Dolovich M, O'Byrne PM. An assessment of the anti‐inflammatory properties of low dose inhaled ciclesonide in asthmatic patients. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California;. 2005:C33; Poster: F64.
-
- Wilson AM, Duong M, Pratt B, Dolovich M, O'Byrne PM. Anti‐inflammatory effects of once daily low dose inhaled ciclesonide in mild to moderate asthmatic patients. Allergy 2006;61(5):537‐42. - PubMed
Zietkowski 2006 {published data only}
-
- Zietkowski Z, Bodzenta‐Lukaszyk A, Tomasiak MM, Szymanski W, Skiepko R. Effect of ciclesonide and fluticasone on exhaled nitric oxide in patients with mild allergic asthma. Respiratory Medicine 2006;100:1651‐6. - PubMed
References to studies excluded from this review
Agertoft 2005 {published data only}
-
- Agertoft L, Pedersen S. Inhaled ciclesonide does not effect lower leg growth rate or HPA‐axis function in children with mild asthma. European Respiratory Journal 2004;24(Suppl 48):377s.
-
- Agertoft L, Pedersen S. Short‐term lower‐leg growth rate and urine cortisol excretion in children treated with ciclesonide. Journal Allergy & Clinical Immunology 2005;115(5):940‐5. - PubMed
-
- Agertorft L, Pedersen S. Lower‐leg growth rate and APA‐axis function in children with asthma during treatment with inhaled ciclesonide. Journal of Allergy and Clinical Immunology 2004;113(2 Suppl):S119.
Bethke 2002 {published data only}
-
- Bethke TD, Drollman A, Hauns B, Nave R, Zech K, Seinijans VW, et al. Bioequivalence of two ciclesonide dosing regimen (4 puffs of 200µg versus 16 puffs of 50µg using a MDI). European Respiratory Journal 2002;20(Suppl 38):109s.
-
- Drollmann A, Bethke TD, Nave R, Zech K, Hauns B, Steinjans VW, et al. Bioequivalence of two ciclesonide dosing regimen (4 puffs of 200µg vs. 8 puffs of 100µg using MDI). European Respiratory Journal 2002;20(Suppl 38):304s.
Dahl 1998 {published data only}
-
- Dahl R, Nielsen LP, Christensen MB, Engelstätter R. Ciclesonide ‐ an inhaled corticosteroid prodrug ‐ inhibits allergen induced early and late phase reactions. European Respiratory Journal 1998;12(Suppl 28):62S.
Derom 2005 {published data only}
-
- Derom E, Velde V, Marissens S, Engelstätter R, Vincken W, Pauwels R. Effects of inhaled ciclesonide and fluticasone propionate on cortisol secretion and airway responsiveness to adenosine 5'monophosphate in asthmatic patients. Pulmonary Pharmacology & Therapeutics 2005;18(5):328‐36. - PubMed
-
- Derom E, Velde V, Marissens S, Vincken W, Pauwels RA. Efficacy and systemic effects of ciclesonide and fluticasone in asthma patients. European Respiratory Journal 2001;18(Suppl 33):147s.
-
- Pauwels RA, Derom E, Velde V, Marissens S, Vincken W. Effects of inhaled ciclesonide and fluticasone propionate on cortisol secretion and PC²° for adenosine in asthma patients. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A768.
Drollman 2004 {published data only}
-
- Drollman A, Nave R, Steinijans VW, Bethke TD, Baumgartner E. Equivalent pharmacokinetics of the active metabolite of ciclesonide with and without use of a spacer for inhalation. Journal of Allergy and Clinical Immunology 2004;113(2 Suppl):S120. - PubMed
-
- Nave R, Baumgartner E, Bethkle D, Steinijans W, Drollman A. Equivalent pharmacokinetics of the active metabolite of ciclesonide when using the ciclesonide MDI without a spacer. European Respiratory Journal 2004;24(Suppl 48):583s.
Erin 2005 {published data only}
-
- Erin EM, Engelstätter R, Hellwig M, Hansel TT, Barnes PJ. Ciclesonide decreases exhaled breath nitric oxide and rapidly reduces airway responsiveness to adenosine monophosphate in asthma patients. European Respiratory Journal 2005;26(Suppl 49):1723.
-
- Erin EM, Englestätter R, Hellwig M, Hansel TT, Barnes PJ. Ciclesonide rapidly reduces airway responsiveness to adenosine monophosphate and deceases exhaled breath nitric oxide in patients with asthma. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:D99; Poster: 916.
-
- Erin EM, Hansel TT, Barnes PJ, Schutze J, Hellwig M, Engelstätter R. Ciclesonide rapidly decreases airway responsiveness to adenosine monophosphate, and reduces exhaled nitric oxide in patients with asthma. XIX World Allergy Organization Congress; Munich, Germany. 2005:Abstract 295.
Gauvreau 2005 {published data only}
-
- Gauvreau GM, Boulet LP, Postma DS, Kawayama T, Watson RM, Duong M, et al. Effect of low‐dose ciclesonide on allergen‐induced responses in subjects with mild allergic asthma. Journal of Allergy & Clinical Immunology 2005;116(2):285‐91. - PubMed
-
- Gauvreau GM, Watson RM, Postma DS, Monchy GR, Deschesnes F, Boulet L. Effect of ciclesonide 40 µg and 80 µg on early and late asthmatic reactions, and sputum eosinophils after allergen challenge in patients with mild asthma. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S210.
-
- Kawayama T, Gauveau GM, Watson RM, Killian KJ, Duong M. Effects of inhaled ciclesonide on circulating Th1/Th2 cells in atopic asthmatics after allergen challenge. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B35; Poster: G1.
-
- O'Byrne PM, Postma DS, Smau L, Widmann A, Gauvreau GM, Monchy J, et al. Once daily ciclesonide reduces allergen‐induced early and late asthmatic reaction, and sputum eosinophils in patients with mild asthma. XIX World Allergy Organization Congress; Munich, Germany 2005:Abstract No. 296.
Kanniess 2001 {published data only}
-
- Kanniess F, Richter K, Bohme S, Jorres RA, Magnussen H. Effect of inhaled ciclesonide on airway responsiveness to inhaled AMP, the composition of induced sputum and exhaled nitric oxide in patients with mild asthma. Pulmonary Pharmacology & Therapeutics 2001;14(2):141‐7. - PubMed
Larsen 2003 {published data only}
-
- Larsen BB, Nielsen LP, Engelstatter R, Steinijans V, Dahl R. Effect of ciclesonide on allergen challenge in subjects with bronchial asthma. Allergy 2003;58(3):207‐12. - PubMed
Lee 2004 {published data only}
-
- Fardon TC, Lee DK, Gray RD, Currie GP, Haggart K, Bates CE, Lipworth BJ. Effects of hydrofluoroalkane formulations of ciclesonide 320µg once daily versus fluticasone propionate 220µg twice daily on methacholine hyperresponsiveness in mild‐to‐moderate persistent asthma. Journal of Allergy and Clinical Immunology 2004;113(2 Suppl):S119.
-
- Lee DK, Haggart K, Currie GP, Bates CE, Lipworth BJ. Effects of hydrofluoroalkane formulations of ciclesonide 400 microg once daily versus fluticasone 250 microg twice daily on methacholine hyper‐responsiveness in mild‐to‐moderate persistent asthma. British Journal of Clinical Pharmacology 2004;58(1):26‐33. - PMC - PubMed
Lee 2005 {published data only}
-
- Lee DK, Fardon TC, Bates CE, Haggart K, McFarlane LC, Lipworth BJ. Airway and systemic effects of hydrofluoroalkane formulations of high‐dose ciclesonide and fluticasone in moderate persistent asthma. Chest 2005;127(3):851‐60. - PubMed
-
- Lee DKC, Lipworth BJ. Relative airway and systemic effects of high dose ciclesonide and fluticasone propionate in asthma. Thorax 2004;59(Suppl II):ii11.
Postma 2001 {published data only}
-
- Postma DS, Schlosser N, Sevette C, Martinat Y, Aumann J, Kafe H. Morning and evening administration of inhaled ciclesonide is equally effective in treatment of asthma. European Respiratory Society Annual Congress. 2002:P1913. - PubMed
-
- Postma DS, Sevette C, Martinat Y, Schlosser N, Aumann J, Kafe H. Treatment of asthma by the inhaled corticosteroid ciclesonide given either in the morning or evening. European Respiratory Journal 2001;17(6):1083‐8. - PubMed
Richter 2005 {published data only}
-
- Richter K, Kanniess F, Biberger C, Nave R, Magnussen H. Comparison of the oropharyngeal deposition of inhaled ciclesonide and fluticasone propionate in patients with asthma. Journal of Clinical Pharmacology 2005;45(2):146‐52. - PubMed
Subbarao 2006 {published data only}
-
- Duong M, Subbarao P, Otis J, Adelroth E, Inman M, Pedersen S, et al. Relationship between exercise‐induced bronchoconstriction and sputum eosinophillia during ciclesonide treatment in asthmatics. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B35; Poster: G11.
-
- Subbarao P, Duong M, Adelroth E, Otis J, Obminski G, Inman M, et al. Effect of ciclesonide dose and duration of therapy on exercise‐induced bronchoconstriction in patients with asthma. Journal of Allercy & Clinical Immunology 2006;117(5):1008‐13. - PubMed
-
- Subbarao P, Duong M, Otis J, Obminski G, Inman M, Alderoith E, et al. Ciclesonide attenuates exercise‐induced bronchoconstriction over time in asthmatic subjects. American Thoracic Society 2005 International conference; May 20‐25; San Diego, California. 2005:B35; Poster: G10.
-
- Subbarao P, Duong M, Pedersen S, Eckert J, Hellbardt S, Engelstatter R, et al. Once daily ciclesonide protects against exercise‐induced bronchoconstriction. XIX World Allergy Organization Congress; Munich, Germany. 2005:Abstract 298.
-
- Subbarao PJ, Duong M, Otis J, Obminski G, Adelroth E, Pedersen S, et al. Ciclesonide is effective in protecting against exercise‐induced bronchoconstriction. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S1. - PubMed
Szefler 2005 {published data only}
-
- Szefler S, Rohatagi S, Williams J, Lloyd M, Kundu S, et al. Ciclesonide, a novel inhaled steroid, does not affect hypothalamic‐pituitary‐adrenal axis function in patients with moderate‐to‐severe persistent asthma. Chest 2005;128(3):1104‐14. - PubMed
-
- Szefler SJ, Herron J, Lloyd M, Rohatagi S, Williams JE, Kundu S, et al. High doses of the novel inhaled steroid ciclesonide have no effect on HPA‐axis function in patients with moderate to severe persistent asthma. Journal of Allergy and Clinical Immunology 2003;111(2 Suppl):S216.
Taylor 1999 {published data only}
-
- Jensen MW, Taylor DA, Englestätter R, Kanabar V, O'Connor BJ. A novel inhaled steroid, ciclesonide, reduces airway responsiveness to AMP in a dose‐related manner. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A406. - PubMed
-
- Taylor DA, Jensen MW, Kanabar V, Engelstätter R, Steinijans VW, Barnes PJ, et al. A dose‐dependent effect of the novel inhaled corticosteroid ciclesonide on airway responsiveness to adenosine‐5'‐monophosphate in asthmatic patients. American Journal of Respiratory & Critical Care Medicine 1999;160(1):237‐43. - PubMed
References to ongoing studies
Arshad {published data only}
-
- An assessment of safety and efficacy in treating moderate to severe asthmatics with inhaled Ciclesonide vs Fluticasone. Ongoing study Starting date of trial not provided. Contact author for more information.
Beck {published data only}
-
- Efficacy and safety of ciclesonide administered with or without different spacers in patients with asthma (12 to 75 y). Clinicaltrials.Gov. 2005. Ongoing study Starting date of trial not provided. Contact author for more information.
Beckman {published data only}
-
- Efficacy of ciclesonide inhaled once daily versus other corticosteroids used for treatment of mild asthma in children (4‐11yrs). Clinicaltrials.Gov. 2005. Ongoing study Starting date of trial not provided. Contact author for more information.
Colatruglio {published data only}
-
- Effect of ciclesonide on quality of life in patients with moderate persistent asthma (21 to 65 y). Clinicaltrials.Gov. 2005. Ongoing study Starting date of trial not provided. Contact author for more information.
Dahl {published data only}
-
- Efficacy of ciclesonide versus fluticasone propionate in patients with mild to moderate asthma (12 to 75 y). Clinicaltrials.Gov. 2005. Ongoing study Starting date of trial not provided. Contact author for more information.
Derom {published data only}
-
- Effect of inhaled ciclesonide versus fluticasone propionate in patients with mild to moderate asthma (18 to 65 y). Clinicaltrials.Gov. 2005. Ongoing study Starting date of trial not provided. Contact author for more information.
Dusser {published data only}
-
- Efficacy of ciclesonide and fluticasone propionate in adult patients with moderate and severe persistent asthma (18 to 75 y). Clinicaltrials.Gov. 2005. Ongoing study Starting date of trial not provided. Contact author for more information.
Engelstätter {published data only}
-
- Efficacy of ciclesonide inhaled once daily versus fluticasone propionate inhaled twice daily in children with asthma (4 to 15 y). Clinicaltrials.Gov. 2005. Ongoing study Starting date of trial not provided. Contact author for more information.
Giwa {published data only}
-
- Comparison of inhaled ciclesonide and fluticasone proprionate in moderate to severe asthma patients, well controlled under high doses of inhaled corticosteroids. Ongoing study Starting date of trial not provided. Contact author for more information.
Hansel {published data only}
-
- A 3‐period double‐blind, cross‐over study on the onset of action of inhaled ciclesonide on airway responsiveness to adenosine monophosphate, sputum eosinophils and exhaled breath NO in patients with asthma. Ongoing study Starting date of trial not provided. Contact author for more information.
O'Byrne {published data only}
-
- Efficacy of ciclesonide vs fixed combination of fluticasone propionate/salmeterol vs placebo in patients with mild persistent asthma (12 to 75 y). Clinicaltrials.Gov. 2005.. Ongoing study Starting date of trial not provided. Contact author for more information.
Park {published data only}
-
- Effectiveness of ciclesonide versus budesonide in patients with asthma (18 to 75 y). Clinicaltrials.Gov. 2005. Ongoing study Starting date of trial not provided. Contact author for more information.
Sanofi Aventis {published data only}
-
- Effects of ciclesonide and beclomethasone on lens opacification in adult subjects with moderate to severe persistent asthma. Clinicaltrials.Gov . 2005; Efficacy of ciclesonide vs. placebo administered as once daily or twice daily in patients not treated with inhaled corticosteroid. Clinicaltrials.Gov. 2005; Effects of ciclesonide MDI 50mg/day and 200mg/day (ex‐value) once‐daily on growth in children with mild persistent asthma. Clinicaltrials.Gov. 2005; Sanofi‐Aventis. Dose response study of inhaled ciclesonide (glucocorticosteroid) to patients with persistent asthma. Clinicaltrials.Gov. 2005.. Ongoing study Starting date of trial not provided. Contact author for more information.
Stenton {published data only}
-
- A double‐blind randomised parallel group study comparing the efficacy and safety of 800 and 1000mcg CIC/day in patients with asthma followed by an open long‐term study to assess the safety of CIC in patients with asthma. Ongoing study Starting date of trial not provided. Contact author for more information.
Additional references
Adams 1999
Adams 2000
Adams 2005a
Adams 2005b
-
- Adams NP, Bestall JC, Lasserson TJ, Jones PW, Cates CJ. Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2005, Issue 4. [Art. No.: CD003135. DOI: 10.1002/14651858] - PubMed
Adams 2006
-
- Adams NP, Jones PW. The dose‐response characteristics of inhaled corticosteroids when used to treat asthma: An overview of Cochrane systematic reviews. Respiratory Medicine 2006;100(8):1297‐306. - PubMed
Adams 2007
BGAM 1997
-
- British Thoracic Society, National Asthma Campaign, Royal College of Physicians of London, et al. The British guidelines on asthma management: 1995 review and position statement. Thorax 1997;52(Suppl 1):S1‐21.
BTS/SIGN 2003
Buston 2000
-
- Buston KM, Wood SF. Non‐compliance amongst adolescents with asthma: listening to what they tell us about self‐management. Family Practice 2000;17:134‐8. - PubMed
Consensus 1999
Consensus 2005
Coutts 1992
Eisen 1990
-
- Eisen SA, Miller DK, Woodward RS, Spitznagel E, Przybeck TR. The effect of prescribed daily dose frequency on patient medication compliance. Archives of Internal Medicine 1990;150:1881‐4. - PubMed
Gerritsen 1989
-
- Gerritsen J, Koeter GH, Postma DS, Schouten JP, Krol K. Prognosis of asthma from childhood to adulthood. Amercian Review of Respiratory Disease 1989;140:1325‐30. - PubMed
GINA 1998
-
- National Institutes of Health (NIH), National Heart, Lung and Blood Institute. Global Strategy for Asthma Management and Prevention. NIH publication No. 96‐36598 (document available at: www.ginasthma.com) 1998.
Higgins 2003
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. - PubMed
Malo 1989
-
- Malo JL, Cartier A, Merland N, Ghezzo H, Burek A, Morris J, et al. Four‐times‐a‐day dosing frequency is better than twice‐a‐day regimen in subjects requiring a high‐dose inhaled steroid, budesonide, to control moderate to severe asthma. American Review of Respiratory Disease 1989;140:624‐8. - PubMed
Martin 1982
Mash 2001
-
- Mash B, Bheekie A, Jones PW. Inhaled versus oral steroids for adults with chronic asthma (Cochrane review). Cochrane Database of Systematic Reviews 2001, Issue 1. - PubMed
Powell 2003
Suissa 2000
-
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low‐dose inhaled corticosteroids and the prevention of death from asthma. New England Journal of Medicine 2000;343:332‐6. - PubMed
Suissa 2002
Tattersfield 2002
-
- Tattersfield AE, Knox AS, Britton JR, Hall IP. Asthma. Lancet 2002;360:1313‐20. - PubMed
Toogood 1982
-
- Toogood JH, Baskerville JC, Jennings B, Lefcoe NM, Johannsson SA. Influence of dosing frequency and schedule on the response of chronic asthmatics to the aerosol steroid, budesonide. Journal Allergy and Clinical Immunology 1982;70:288‐98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

