Paliperidone for schizophrenia
- PMID: 18425951
- PMCID: PMC12152845
- DOI: 10.1002/14651858.CD006369.pub2
Paliperidone for schizophrenia
Abstract
Background: Paliperidone, risperidone's active metabolite, is now available in an oral formulation for daily use, and an intramuscular formulation for monthly administration may follow shortly.
Objectives: To compare effects of oral paliperidone with any other treatment for people with schizophrenia and schizophrenia-like illnesses.
Search strategy: We searched the Cochrane Schizophrenia Group's Register (December 2006), and inspected references of identified studies for further trials. We contacted the manufacturers of paliperidone, the Food and Drug Administration, and authors of relevant trials for additional material.
Selection criteria: We included all relevant randomised trials.
Data collection and analysis: We independently selected and critically appraised studies, extracted data and analysed on an intention-to-treat basis. Where possible and appropriate, we calculated risk ratios (RR) and their 95% confidence intervals (CI) with the number needed to treat (NNT). We calculated Weighted Mean Differences (WMD) for continuous data.
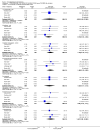
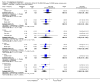
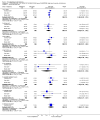
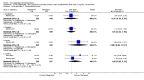
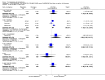
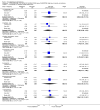
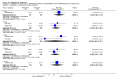
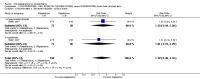
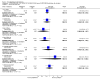
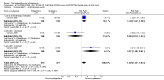
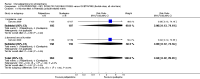
Main results: Five studies compared paliperidone with placebo. Fewer people left the studies early if they were randomized to paliperidone (n=1647, 5 RCTs, RR 0.68 CI 0.61 to 0.76, NNT 7 CI 6 to 9) and those receiving any dose of paliperidone were significantly more likely to have an improvement in global state (n=1420, 4RCTs, RR 0.69 CI 0.63 to 0.75, NNT 5 CI 4 to 6). People randomised to paliperidone were less likely to experience a recurrence of psychosis (n=1638, 5 RCTs, RR 0.45 CI 0.31 to 0.66, NNT 16 CI 13 to 26) than those allocated to placebo. Adverse effect data were not well reported but paliperidone does seem to produce a greater incidence of tachycardia than placebo (n=1638, 5 RCTs, RR1.88 CI 1.28 to 2.76, NNH 21 CI 11 to 90) and a consistent, significant elevation in serum prolactin was found for both men (n=413, 3 RCTs, WMD 27.68 CI 23.66 to 31.69) and women (n=252, 3 RCTs, WMD 87.39 CI 74.27 to 100.51). People receiving paliperidone were more likely to experience extrapyramidal disorders (n=1638, 5 RCTs, RR 2.21 CI 1.26 to 3.88, NNH 28 CI 12 to 129) and weight gain (n=769, 4 RCTs, WMD 1.07 CI 0.65 to 1.49, I-squared 78%) compared with those allocated to placebo. When compared with 10 mg/day olanzapine we found no differences between paliperidone and olanzapine for leaving in the short term (n=1332, 3 RCTs, RR 1.04 CI 0.89 to 1.21; 40% in both groups left by six weeks). Those receiving any dose of paliperidone were no more likely to have a recurrence of psychotic symptoms than those receiving 10 mg/day olanzapine (n=1327, 3 RCTs, RR 0.1.07 CI 0.64 to 1.76). Data from all three studies found paliperidone was less likely to produce a weight change than olanzapine (n=660, 3 RCTs, WMD -0.88 CI -1.38 to -0.37). Results for various movement disorders all favoured olanzapine. There are no clear data relating to social functioning, services use, quality of life, satisfaction and cost.
Authors' conclusions: In short-term studies, oral paliperidone is an antipsychotic that is more efficacious than placebo. We found its adverse effects to be similar to those of its parent compound, risperidone, with movement disorders, weight gain, and tachycardia all more common with paliperidone than placebo. In addition, paliperidone is associated with substantial increases in serum prolactin that may be associated with sexual dysfunction, although sexual functioning outcomes were not reported. At doses greater than 3 mg per day, oral paliperidone appears comparable in efficacy to oral olanzapine 10 mg per day. Regarding the critical comparison of oral paliperidone to risperidone, we have no information and are thus unable to determine if paliperidone has any advantages or disadvantages compared to its well-known parent compound.
Conflict of interest statement
The authors received no financial consideration from any parties for the preparation of this review. Dr. Stroup has been a consultant for AstraZeneca, Janssen, Lilly, and Pfizer, and has spoken at events sponsored by Lilly, Lundbeck, and Pfizer.
Figures
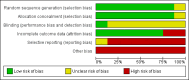



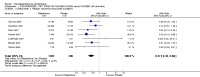










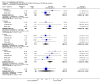







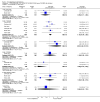










































Comment in
-
Review: paliperidone reduces symptoms of schizophrenia and schizophrenia-like illness.Evid Based Ment Health. 2008 Nov;11(4):114. doi: 10.1136/ebmh.11.4.114. Evid Based Ment Health. 2008. PMID: 18952963 No abstract available.
References
References to studies included in this review
Canuso 2009 {published and unpublished data}
-
- Canuso C, Carothers J, Dirks B, Zhu Y, Kosik‐Gonzalez C. A double‐blind placebo‐controlled trial comparing paliperidone ER and quetiapine in patients with a recent acute exacerbation of schizophrenia. Proceedings of the 161st Annual Meeting of the American Psychiatric Association; 2008 May 3‐6; Washington DC. Washington, DC, 2008.
-
- Canuso C, Carothers J, Dirks B, Zhu Y, Schreiner A, Kosik‐Gonzalez C. A double‐blind placebo‐controlled trial comparing paliperidone er and quetiapine in patients with a recent acute exacerbation of schizophrenia. Schizophrenia Research 2008;102(Suppl 2):252.
-
- Canuso C, Dirks B, Carothers J, Zhu T, Kosik‐Gonzalez C. A double‐blind, placebo‐controlled trial of paliperidone er and quetiapine in patients with a recent acute exacerbation of schizophrenia. Proceedings of the 20th Annual U.S. Psychiatric & Mental Health Congress; 2007 11‐14 Oct; Orlando, Florida. Orlando, Florida, 2007.
-
- Canuso C, Dirks B, Carothers J, Zhu Y, Kosik‐Gonzalez C. A comparative analysis of paliperidone er and quetiapine in patients with a recent, acute exacerbation of schizophrenia. Schizophrenia Research 2008;98:158‐9. [DOI: 10.1016/j.schres.2007.12.372] - DOI
-
- Canuso CM, Dirks B, Carothers J, Kosik‐Gonzalez C, Bossie CA, Zhu Y, Damaraju CV, Kalali AH, Mahmoud R. Randomized, double‐blind, placebo‐controlled study of paliperidone extended‐release and quetiapine in inpatients with recently exacerbated schizophrenia. American Journal of Psychiatry 2009;116(6):691‐701. [DOI: 10.1176/appi.ajp.2009.08040613; PUBMED: 19411369] - DOI - PubMed
Cleton 2007 {published data only (unpublished sought but not used)}
-
- Cleton A, Rossenu S, Talluri K, Francetic I, Remmerie B, Janssens L, Eerdekens M, Boom S. Evaluation of the pharmacokinetics of an extended‐release formulation of paliperidone with an immediate‐release formulation of risperidone. Clinical Pharmacology and Therapeutics 2007;81(Suppl 1):S62.
-
- Cleton A, Rossenu S, Talluri K, Francetic I, Remmerie B, Janssens L, Eerdekens M, Boom S. Comparison of pharmacodynamic parameters resulting from administration of an paliperidone extended‐release tablet formulation and an immediate‐release formulation of risperidone in subjects with schizophrenia. Clinical Pharmacology and Therapeutics 2007;81(Suppl 1):S61‐2.
-
- Eerdekens M, Kramer M, Rossenu S, Pandina G, Boom S, Francetic I, Janssens L, Cleton A. Effect of paliperidone extended‐release tablets on prolactin exposure in patients with stable schizophrenia (Poster no. 335). Proceedings of the USP and MHC; 2006 16‐19 Nov; New Orleans, USA. New Orleans, USA, 2006.
Davidson 2007 {published and unpublished data}
-
- Davidson M, Emsley R, Kramer M, Ford L. Efficacy, safety and effect on functioning of paliperidone extended‐release tablets in schizophrenia: an international 6‐week placebo‐controlled study. Proceedings of the 13th Biennial Winter Workshop on Schizophrenia; 2006 Feb 4‐10; Davos, Switzerland. 2006.
-
- Davidson M, Emsley R, Kramer M, Ford L, Pan G, Lim P, Eerdekens M. Efficacy, safety and early response of paliperidone extended‐release tablets (paliperidone ER): results of a 6‐week, randomized, placebo‐controlled study. Schizophrenia Research 2007;93(1‐3):117‐30. [DOI: 10.1016/j.schres.2007.03.003; MEDLINE: ] - DOI - PubMed
-
- Davidson M, Emsley R, Kramer M, Gassmann‐Mayer C, Lim P, Pan J, Eerdekens M. Efficacy, safety and effect on functioning of oral paliperidone extended‐release tablets in the acute treatment of schizophrenia: an international 6‐week placebo‐controlled study. Schizophrenia Research 2006;81(Suppl 1):43.
-
- Janssen‐Cilag Ltd. Data on File PCDoF2. Communication from Janssen‐Cilag Ltd.
Kane 2007 {published and unpublished data}
-
- Janssen‐Cilag Ltd. Data on File PCDoF2. Communication from Janssen‐Cilag Ltd.
-
- Kane J, Canas F, Kramer M, Ford L, Gassmann‐Mayer C, Lim P, Eerdekens M. Treatment of schizophrenia with paliperidone extended‐release tablets: a 6‐week placebo ‐controlled trial. Schizophrenia Research 2007;90(3):147‐61. [MEDLINE: ] - PubMed
-
- Kane J, Kramer M, Ford L, Gassmann‐Mayer C, Lim P, Eerdekens M. Patients with acute schizophrenia: treatment with three fixed dosages of oral paliperidone extended‐release tablets in an international 6‐week placebo‐controlled study. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25; Toronto, Canada. 2006.
-
- Kane J, Kramer M, Ford L, Gassmann‐Mayer C, Lim P, Eerdekens M. Treatment of schizophrenia using oral paliperidone extended‐release tablets: a 6‐week placebo‐controlled study. Proceedings of the 13th Biennial Winter Workshop on Schizophrenia; 2006 Feb 4‐10; Davos, Switzerland. 2006.
Kramer 2007 {published and unpublished data}
-
- Kramer M, Kushner S, Vijapurkar U, Lim P, Eerdekens M. Delaying symptom recurrence in patients with schizophrenia treated with paliperidone extended‐release tablets. Journal of the European College of Neuropsychopharmacology 2006;16(Suppl 4):s386.
-
- Kramer M, Simpson G, Maciulis V, Kushner S, Vijapurkar U, Lim P, Eerdekens M. Paliperidone extended‐release tablets for prevention of symptom recurrence in patients with schizophrenia: a randomized, double‐blind, placebo‐controlled study. Journal of Clinical Psychopharmacology 2007;27(1):6‐14. [MEDLINE: ; NCT00086320] - PubMed
Luthringer 2007 {published and unpublished data}
-
- Luthringer R, Staner L, Noel N, Muzet M, Gassmann‐Mayer C, Talluri K, Cleton A, Eerdekens M, Battisti W, Palumbo J. A double‐blind, placebo‐controlled, randomized study evaluating the effect of paliperidone extended‐release tablets on sleep architecture in patients with schizophrenia. International Clinical Psychopharmacology 2007;22(5):299‐308. [DOI: 10.1097/YIC.0b013e3281c55f4f; PUBMED: 17690599] - DOI - PubMed
Marder 2007 {published and unpublished data}
-
- Janssen‐Cilag Ltd. Data on File PCDoF2. Communication from Janssen‐Cilag Ltd.
-
- Marder S, Kramer M, Ford L, Eerdekens E, Lim P, Eerdekens M. A 6‐week, US‐based placebo‐controlled study on the efficacy and tolerability of two fixed dosages of oral paliperidone extended‐release tablets in the treatment of acute schizophrenia. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25; Toronto, Canada. 2006.
-
- Marder S, Kramer M, Ford L, Eerdekens E, Lim P, Eerdekens M. A 6‐week, US‐based placebo‐controlled study on the efficacy and tolerability of two fixed dosages of oral paliperidone extended‐release tablets in the treatment of acute schizophrenia. Schizophrenia Research 2006;81(Suppl 1):56.
Tzimos 2008 {published and unpublished data}
-
- Tzimos A, Kramer M, Ford L, Gassmann‐Mayer C, Lim P, Eerdekens M. A 6‐week placebo‐controlled study of the safety and tolerability of flexible doses of oral paliperidone extended‐release tablets in the treatment of schizophrenia in elderly patients. Proceedings of the 25th Collegium Internationale Neuro‐psychopharmacologicum; 2006 Jul 9‐13; Chicago, Illinois. 2006.
-
- Tzimos A, Kramer M, Ford L, Gassmann‐Mayer C, Lim P, Eerdekens M. A 6‐week placebo‐controlled study on the safety and tolerability of flexible doses of oral paliperidone extended‐release tablets in the treatment of schizophrenia in elderly patients. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25; Toronto, Canada. 2006.
-
- Tzimos A, Kramer M, McLemore J, Ford L, Gassmann‐Mayer C, Lim P, Eerdekens M. A 6‐week placebo‐controlled study of the safety and tolerability of flexible doses of oral paliperidone extended‐release tablets in the treatment of schizophrenia in elderly patients. Schizophrenia Bulletin 2007;33(Suppl 2):s464.
-
- Tzimos A, Samokhvalov V, Kramer M, Ford L, Gassmann‐Mayer C, Lim P, Eerdekens M. Safety and Tolerability of Oral Paliperidone Extended‐Release Tablets in Elderly Patients With Schizophrenia: A Double‐Blind, Placebo‐Controlled Study With Six‐Month Open‐Label Extension. American Journal of Geriatric Psychiatry 2008;16(1):31‐43. [PUBMED: 18165460] - PubMed
References to studies excluded from this review
Arakawa 2008 {published data only}
-
- Arakawa R, Ito H, Takano A, Takahashi H, Morimoto T, Sassa T, Ohta K, Kato M, Okubo Y, Suhara T. Dose‐finding study of paliperidone ER based on striatal and extrastriatal dopamine D(2) receptor occupancy in patients with schizophrenia. Psychopharmacology (Berl) 2008;197(2):229‐35. [DOI: 10.1007/s00213-007-1029-z; MEDLINE: ] - DOI - PubMed
Borison 1994 {published data only}
-
- Borison R, Diamond B, Pathiraja A, Meibach R. Pharmacokinetics of risperidone in chronic schizophrenic patients. Psychopharmacology Bulletin 1994;30(2):193‐7. [MEDLINE: ] - PubMed
Cleton 2006 {published data only}
-
- Cleton A, Talluri K, Leempoels A, Thyssen A, Janssens L, Eerdekens M, Boom S. No pharmacokinetic interaction between trimethoprim and paliperidone ER in healthy subjects. Clinical pharmacology and therapeutics 2006;79(2):22.
Cleton 2007b {published data only}
-
- Cleton A, Rossenu S, Boom S, Remmerie B, Alnabawy A, Talluri K, Eerdekens M. Evaluation of the pharmacokinetics of paliperidone extended‐release tablets in healthy elderly subjects. Clinical pharmacology and therapeutics 2007;81(Suppl 1):S62.
References to ongoing studies
Armenteros 2001 {published data only}
-
- Armenteros J. Risperidone treatment of adolescents with schizophrenia. CRISP database issue 2001. [CRISP Grant Number 5R29MH57084‐04]
Rossenu 2008 {published data only (unpublished sought but not used)}
-
- Rossenu S, Cleton A, Talluri K, Francetic I, Canuso C, Remmerie B, Janssens L, Eerdekens M, Boom S. Pharmacodynamics of paliperidone extended‐release tablets and immediate‐release risperidone in schizophrenia. Schizophrenia Research 2008;98:159.
Additional references
Altman 1996
Angelucci 2009
-
- Angelucci F, Ricci V, Martinotti G, Caltagirone C, Bria P. Paliperidone for treatment of obsessive‐compulsive resistant symptoms in schizophrenia: A case report. Prog Neuropsychopharmacol Biol Psychiatry 2009. [PUBMED: 19555731] - PubMed
Anonymous 2007
-
- Anonymous. Molecule of the month. Paliperidone. Drug News Perspective 2007;20(5):333. [MEDLINE: ] - PubMed
Anonymous 2007b
-
- Anonymous. Paliperidone: new drug. Just a metabolite of risperidone, a neuroleptic soon off‐patent. Prescrire International 2007;16(92):236‐7. [PUBMED: 18092400] - PubMed
Arakawa 2008
-
- Arakawa R, Ito H, Takano A, Takahashi H, Morimoto T, Sassa T, Ohta K, Kato M, Okubo Y, Suhara T. Dose‐finding study of paliperidone ER based on striatal and extrastriatal dopamine D2 receptor occupancy in patients with schizophrenia. Psychopharmacology 2008;197(2):229‐35. [PUBMED: 18058087] - PubMed
Aravagiri 1998a
-
- Aravagiri M, Yuwiler A, Marder S. Distribution after repeated oral administration of different dose levels of risperidone and 9‐hydroxy‐risperidone in the brain and other tissues of rat. Psychopharmacology 1998;139(4):356‐63. [MEDLINE: ] - PubMed
Aravagiri 1998b
-
- Aravagiri M, Marder S, Wirshing D, Wirshing W. Plasma concentrations of risperidone and its 9‐hydroxy metabolite and their relationship to dose in schizophrenic patients: simultaneous determination by a high performance liquid chromatography with electrochemical detection. Pharmacopsychiatry 1998;31(3):102‐9. [MEDLINE: ] - PubMed
Aravagiri 2000
-
- Aravagiri M, Marder S. Simultaneous determination of risperidone and 9‐hydroxyrisperidone in plasma by liquid chromatography/electrospray tandem mass spectrometry. Journal of Mass Spectrometry 2000;35(6):718‐24. [MEDLINE: ] - PubMed
Aravagiri 2002
-
- Aravagiri M, Marder S. Brain, plasma and tissue pharmacokinetics of risperidone and 9‐hydroxyrisperidone after separate oral administration to rats. Psychopharmacology 2002;159(4):424‐31. [MEDLINE: ] - PubMed
Aravagiri 2003
-
- Aravagiri M, Marder S, Nuechterlein K, Gitlin M. Intra‐ and interindividual variations in steady‐state plasma concentrations of risperidone and 9‐hydroxyrisperidone in schizophrenic patients treated chronically with various doses of risperidone. Therapeutic Drug Monitoring 2003;25(6):657‐64. [MEDLINE: ] - PubMed
Awouters 2007
-
- Awouters FH, Lewi PJ. Forty years of antipsychotic Drug research from haloperidol to paliperidone with Dr. Paul Janssen. Arzneimittelforschung 2007;57(10):625‐32. [MEDLINE: ] - PubMed
Barnes 1989
-
- Barnes TR. A rating scale for drug‐induced akathisia. British Journal of Psychiatry 1989;154:672‐6. [MEDLINE: ] - PubMed
Bland 1997
Bostwick 2009
-
- Bostwick JR, Guthrie SK, Ellingrod VL. Antipsychotic‐induced hyperprolactinemia. Pharmacotherapy 2009;29(1):64‐73. [PUBMED: 19113797] - PubMed
Byerly 2007
-
- Byerly M, Suppes T, Tran QV, Baker RA. Clinical implications of antipsychotic‐induced hyperprolactinemia in patients with schizophrenia spectrum or bipolar spectrum disorders: Recent developments and current perspectives. Journal of Clinical Psychopharmacology 2007;27(6):639‐61. [MEDLINE: ] - PubMed
Canuso 2008
-
- Canuso CM, Youssef EA, Bossie CA, Turkoz I, Schreiner A, Simpson GM. Paliperidone extended‐release tablets in schizophrenia patients previously treated with risperidone. International Clinical Psychopharmacology 2008;23(4):209‐15. [PUBMED: 18545059] - PubMed
Canuso 2009a
-
- Canuso CM, Turkoz I, Sheehanssie JJ, Bo CA. Efficacy and safety of paliperidone extended‐release in schizophrenia patients with prominent affective symptoms. Journal of Affective Disorders 2009. [PUBMED: 19552963] - PubMed
Canuso 2009b
-
- Canuso CM, Bossie CA, Turkoz I, Alphs L. Paliperidone extended‐release for schizophrenia: Effects on symptoms and functioning in acutely ill patients with negative symptoms. Schizophrenia Research 2009. [PUBMED: 19560322] - PubMed
Citrome 2007
-
- Citrome L. Paliperidone: quo vadis?. International Journal of Clinical Practice 2007;61(4):653‐62. [MEDLINE: ] - PubMed
Cohrs 2008
-
- Cohrs S. Sleep disturbances in patients with schizophrenia : Impact and effect of antipsychotics. CNS Drugs 2008;22(11):939‐62. [PUBMED: 18840034] - PubMed
Danel 2008
-
- Danel C, Azaroual N, Brunel A, Lannoy D, Vermeersch G, Odou P, Vaccher C. Study of the complexation of risperidone and 9‐hydroxyrisperidone with cyclodextrin hosts using affinity capillary electrophoresis and (1)H NMR spectroscopy. Journal of chromatography 2008;1215(1‐2):185‐93. [PUBMED: 19013582] - PubMed
Deeks 2005
-
- Deeks JJ, Higgins JPT, Altman DG. Analysing and presenting results. Section 8: The Cochrane Library. In Deeks JJ, Higgins JPT, Altman DG (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 [updated September 2008] The Cochrane Collaboration. Chichester, UK: John Wiley & Sons, Ltd, 2008.
Dirks 2006
-
- Dirks B, Canuso C, Eerdekens M, Turkoz I, Alberio R, Gharabawi G. Efficacy of paliperidone extended‐release tablets in patients with schizophrenia and predominant negative symptoms. International Journal of Neuropsychopharmacology. 2006; Vol. 9:162. [DOI: 10.1017/S1461145706007292] - DOI
Divine 1992
-
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. [PUBMED: 1453246] - PubMed
Dlugosz 2007
-
- Dlugosz H, Nasrallah HA. Paliperidone: a new extended‐release oral atypical antipsychotic. Expert Opinion on Pharmacotherapy 2007;8(14):2307‐13. [PUBMED: 17927485] - PubMed
Dolder 2008
-
- Dolder C, Nelson M, Deyo Z. Paliperidone for schizophrenia. American Journal of Health‐System Pharmacy 2008;65(5):403‐13. [PUBMED: 18281731] - PubMed
Donner 2002
-
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21(19):2971‐80. [PUBMED: 12325113] - PubMed
Dopheide 2008
-
- Dopheide JA. Paliperidone: an improvement over risperidone?. American Journal of Health‐System Pharmacy 2008;65(5):401. [PUBMED: 18281730] - PubMed
Duggal 2007
-
- Duggal HS. Possible neuroleptic malignant syndrome associated with paliperidone. Journal of Neuropsychiatry Clinical Neuroscience 2007;19(4):477‐8. [MEDLINE: ] - PubMed
Duval 2008
-
- Duval F, Guillon MS, Mokrani MC, Crocq MA, Garcia Duarte F. Relationship between prolactin secretion, and plasma risperidone and 9‐hydroxyrisperidone concentrations in adolescents with schizophreniform disorder. Psychoneuroendocrinology 2008;33(2):255‐9. [PUBMED: 18053652] - PubMed
Edwards 2008
-
- Edwards NC, Pesa J, Meletiche DM, Engelhart L, Thompson AK, Sherr J, Dirani R. One‐year clinical and economic consequences of oral atypical antipsychotics in the treatment of schizophrenia. Current Medical Research Opinion 2008;24(12):3341‐55. [PUBMED: 18954497] - PubMed
Egger 1997
Elbourne 2002
-
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials:methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [PUBMED: 11914310] - PubMed
Emsley 2008
-
- Emsley R, Berwaerts J, Eerdekens M, Kramer M, Lane R, Lim P, Hough D, Palumbo J. Efficacy and safety of oral paliperidone extended‐release tablets in the treatment of acute schizophrenia: pooled ate from three 52‐week open‐label studies. International Clinical Psychopharmacology 2008;23(6):343‐56. [PUBMED: 18854723] - PubMed
Feng 2008
-
- Feng Y, Pollock BG, Coley K, Marder S, Miller D, Kirshner M, Aravagiri M, Schneider L, Bies RR. Population pharmacokinetic analysis for risperidone using highly sparse sampling measurements from the CATIE study. British Journal of Clinical Pharmacology 2008;66(5):629‐39. [PUBMED: 18771484] - PMC - PubMed
Fitzgerald 2008
-
- Fitzgerald P, Dinan TG. Prolactin and dopamine: what is the connection? A review article. Journal of Psychopharmacology 2008;22(Suppl 2):12‐9. [PUBMED: 18477617] - PubMed
Fowler 2008
-
- Fowler JA, Bettinger TL, Argo TR. Paliperidone extended‐release tablets for the acute and maintenance treatment of schizophrenia. Clinical Therapeutics 2008;30(2):231‐48. [PUBMED: 18343262] - PubMed
Freedman 2003
-
- Freedman R. Schizophrenia. New England Journal of Medicine 2003;349(18):1738‐49. - PubMed
Freudenmann 2009
-
- Freudenmann RW, Kühnlein P, Lepping P, Schönfeldt‐Lecuona C. Secondary delusional parasitosis treated with paliperidone. Clinical and Experimental Dermatology 2009;34(3):375‐7. [PUBMED: 19040517] - PubMed
Friberg 2009
Gagnon 2006
-
- Gagnon D, Adriaenssen I, Nasrallah H, Morosini P. Reliability, validity and sensitivity to change of the personal and social performance scale in patients with stable schizophrenia. International Journal of Neuropsychopharmacology 2006;9(Suppl 1):288.
Geitona 2008
-
- Geitona M, Kousoulakou H, Ollandezos M, Athanasakis K, Papanicolaou S, Kyriopoulos I. Costs and effects of paliperidone extended release compared with alternative oral antipsychotic agents in patients with schizophrenia in Greece: A cost effectiveness study. Annals of General Psychiatry 2008;7:16‐28. [PMCID: PMC2553072; PUBMED: 18755025] - PMC - PubMed
Gilbody 2000
Glick 2009
-
- Glick ID, Bossie CA, Alphs L, Canuso CM. Onset and persistence of antipsychotic response in patients with schizophrenia. Journal of Clinical Psychopharmacology 2009;29(6):542‐7. [PUBMED: 19910718] - PubMed
Gulliford 1999
-
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149(9):876‐83. - PubMed
Guy 1976
-
- Guy W. Early clinical drug evaluation (ECDEU) assessment manual for psychopharmacology. Washington DC: National Institute of Mental Health, 1976.
Heinrichs 1984
-
- Heinrichs DW, Hanlon ET, Carpenter WT. The quality of life scale: an instrument for rating the schizophrenic deficit syndrome. Schizophrenia Bulletin 1984;10(3):388‐96. [MEDLINE: ] - PubMed
Higgins 2005
-
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 [updated September 2008]. The Cochrane Collaboration. Chichester, UK: John Wiley & Sons, Ltd, 2008.
Hogarty 1974
-
- Hogarty GE, Goldberg SC, Schooler NR, Ulrich RF. Drug and sociotherapy in the aftercare of schizophrenic patients: two‐year relapse rates. Archives of General Psychiatry 1974;31:603‐8. - PubMed
Hosalli 2003
Hough 2009
-
- Hough D, Nuamah IF, Lim P, Sampson A, Gagnon DD, Rothman M. Independent effect of paliperidone extended release on social functioning beyond its effect on positive and negative symptoms of schizophrenia: a mediation analysis. Journal of Clinical Psychopharmacology 2009;29(5):496‐7. [PUBMED: 19745652] - PubMed
Hunter 2003
Hussar 2008
-
- Hussar DA. New drug update: 2007. The Consultant Pharmacist 2008;23(4):276‐96. [PUBMED: 18454587] - PubMed
Huther 2008
-
- Huther R, Gebhart C, Mirisch S, Bauml J, Forstl H. Choreatic symptoms during and after treatment with paliperidone and escitalopram. Pharmacopsychiatry 2008;41(5):203‐4. [PUBMED: 18763225] - PubMed
Ilett 2004
-
- Illett KF, Hackett LP, Kristensen JH, Vaddadi KS, Gardiner SJ, Begg EJ. Transfer of risperidone and 9‐hydroxyrisperidone into human milk. Annals of Pharmacotherapy 2004;38(2):273‐6. [PUBMED: 14742766] - PubMed
Janicak 2006
-
- Janicak PG, Davis JM, Preskorn SH, Ayd FJ, Marder SR, Pavuluri MN. Principles and Practice of Psychopharmacology. 4th Edition. Philadelphia: Lippincott Williams and Wilkins, 2006. [0781760577]
Janicak 2008a
-
- Janicak PG, Wu JH, Mao L. Hospitalization rates before and after initiation of paliperidone ER in patients with schizophrenia: results from open‐label extensions of the US double‐blind trials. Current Medical Research and Opinion 2008;24(6):1807‐15. [PUBMED: 18559166] - PubMed
Janicak 2008b
-
- Janicak PG, Jasmanda HW, Amatniek J, Mao L, Pesa J, Trott J, Kozma C, Canuso CM. Changes in mental health resource use after initiation of paliperidone ER in patients with schizophrenia. Proceedings of the Institute of Psychiatrics Services Annual Meeting; 2008 Oct 2‐5; Chicago. Chicago, 2008.
Janssen 2003
-
- Janssen Pharmaceutica Products LP. Risperdal Consta (risperidone) long‐acting injection prescribing information. Janssen Pharmaceutica Products, L.P. 2003.
Janssen 2006
-
- Janssen Pharmaceutica Products LP. Invega (paliperidone) extended‐release tablets prescribing information. Janssen Pharmaceutica Products, L.P. 2006.
Janssen‐Cilag 1996
-
- Janssen‐Cilag Ltd. Risperidal (risperidone) summary of product characteristics. UK: Janssen‐Cilag Ltd 1996.
Jayaram 2006
Jones 2008
-
- Jones A. What are the nursing implications when using paliperidone prolonged release for people with schizophrenia. Journal of Psychiatric and Mental Health Nursing 2008;15(10):792‐9. [PUBMED: 19012670] - PubMed
Jones 2008a
-
- Jones MP, Nicholl D, Trakas K. Efficacy‐tolerability profiles of oral atypical antipsychotics for schizophrenia: a meta‐analysis including paliperidone extended‐release. Proceedings of the 11th Annual European Conference of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR); 2008 Nov 8‐11; Athens, Greece. Athens, Greece, 2008.
Kapur 1995
-
- Kapur S, Remington G, Zipursky RB, Wilson AA, Houle S. The D2 dopamine receptor occupancy of risperidone and its relationship to extrapyramidal symptoms: a PET study. Life Science 1995;57(10):103‐7. [MEDLINE: ] - PubMed
Karlsson 2006
-
- Karlsson P, Dencker E, Nyberg S, Cselenyi Z, Mannaert E, Boom S, Talluri K, Rossenu S, Eriksson B, Eerdekens M, Farde L. Pharmacokinetics and dopamine D2 and serotonin 5‐HT2A receptor occupancy of paliperidone in healthy subjects. Schizophrenia Research 2006;81(Suppl 1):85‐6.
Kay 1986
-
- Kay S, Opler L, Fizbein A. The positive and negative syndrome scale (PANSS) manual. North Tohawanda, NY: Multi‐Health System, 1986.
Knegtering 2005
-
- Knegtering R, Baselmans P, Castelein S, Bosker F, Bruggeman R, Bosch R. Predominant role of the 9‐hydroxy metabolite of risperidone in elevating blood prolactin levels. American Journal of Psychiatry 2005;162(5):1010‐2. [MEDLINE: ] - PubMed
Knight 2009
-
- Knight JA, Smith C, Toohey N, Klein MT, Teitler M. Pharmacological analysis of the novel, rapid, and potent inactivation of the human 5‐Hydroxytryptamine7 receptor by risperidone, 9‐OH‐Risperidone, and other inactivating antagonists. Molecular Pharmacology 2009;75(2):374‐80. [PUBMED: 18996971] - PMC - PubMed
Kostic 2006
-
- Kostic D, Bossie C, Turkoz I, Bouhours P, Canuso C. Paliperidone extended‐release tablets in patients recently diagnosed with schizophrenia. International Journal of Neuropsychopharmacology 2006;9:161. [DOI: 10.1017/S1461145706007292] - DOI
Kramer 2008
-
- Kramer M, Eerdekens M, Lane R, Schreiner A, Lim P, Sherr J, Palumbo J. Oral paliperidone extended‐release treatment: long‐term metabolic outcomes in patients with schizophrenia. Schizophrenia Research 2008;98:173‐4. [DOI: 10.1016/j.schres.2007.12.408] - DOI
Lautenschlager 2008
-
- Lautenschlager M, Heinz A. Paliperidone‐ER: first atypical antipsychotic with oral extended release formulation. Expert Review of Neurotherapeutics 2008;8(2):193‐200. [PUBMED: 18271706] - PubMed
Leon 2007
-
- Leon J, Susce MT, Pan RM, Wedlund PJ, Orrego ML, Diaz JF. A study of genetic (CYP2D6 and ABCB1) and environmental (drug inhibitors and inducers) variables that may influence plasma risperidone levels. Pharmacopsychiatry 2007;40(3):93‐102. [MEDLINE: ] - PubMed
Leucht 2005
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale scores. The British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] - PubMed
Leucht 2005a
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia research 2005;79(2‐3):231‐8. [PUBMED: 15982856] - PubMed
Lieberman 2005
-
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 2005;353(12):1209‐23. [MEDLINE: ] - PubMed
Lieberman 2006
-
- Lieberman JA, Stroup TS, Perkins DO. The American Psychiatric Publishing Textbook of Schizophrenia. Washington, DC: American Psychiatric Publishing, 2006. [ISBN 1585621919]
Mackay 1998
-
- Mackay FJ, Wilton LV, Pearce GL, Freemantle SN, Mann RD. The safety of risperidone: a post‐marketing study on 7684 patients. Human Psychopharmacology 1998;13:413‐8.
Mannens 1993
-
- Mannens G, Huang M‐L, Meuldermans W, Hendrickx J, Woestenborghs R, Heykants J. Absorption, metabolism, and excretion of risperidone in humans. Drug Metabolism and Disposition 1993;21(6):1134‐41. - PubMed
Marder 1997
-
- Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: Combined results of the North American trials. Journal of Clinical Psychiatry 1997;58(12):538‐46. [MEDLINE: ] - PubMed
Marino 2008
-
- Marino J, Caballero J. Paliperidone extended‐release for the treatment of schizophrenia. Pharmacotherapy 2008;28(10):1283‐98. [PUBMED: 18823223] - PubMed
Marshall 2000
-
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:259‐62. - PubMed
Melkersson 2006
-
- Melkersson K. Prolactin elevation of the antipsychotic risperidone is predominantly related to its 9‐hydroxy metabolite. Human Psychopharmacology 2006;21(8):529‐32. [MEDLINE: ] - PubMed
Meltzer 2008
-
- Meltzer HY, Bobo WV, Nuamah IF, Lane R, Hough D, Kramer M, Eerdekens M. Efficacy and tolerability of oral paliperidone extended‐release tablets in the treatment of acute schizophrenia: pooled data from three 6‐week, placebo‐controlled studies. Journal of Clinical Psychiatry 2008;69(5):817‐29. [PUBMED: 18466043] - PubMed
Moher 2001
-
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285:1987‐91. - PubMed
Nasrallah 2008
-
- Nasrallah HA. Atypical antipsychotic‐induced metabolic side effects: insight from receptor‐binding profiles. Molecular Psychiatry 2008;13(1):27‐35. [PUBMED: 17848919] - PubMed
Nussbaum 2008
Overall 1962
-
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799‐812.
Pandina 2007
-
- Pandina GJ, Canuso CM, Youssef E, Kujawa M, Mahmoud R. Limitations in discerning the effects of risperidone and its 9‐hydroxy metabolite on prolactin levels in a small study of patients with schizophrenia. Human Psychopharmacology 2007;22(5):326‐7. [PUBMED: 17599334] - PubMed
Pani 2009
-
- Pani L, Marchese G. Expected clinical benefits of paliperidone extended‐release formulation when compared with risperidone immediate‐release. Expert Opinion On Drug Delivery 2009;6(3):319‐31. [PUBMED: 19317589] - PubMed
Patrick 2006
-
- Patrick D, Adriaenssen I, Morosini P, Rothman M. Reliability, validity and sensitivity to change of the personal and social performance scale in patients with acute schizophrenia. International Journal of Neuropsychopharmacology 2006;9(Suppl 1):S287‐88.
Patrick 2009
-
- Patrick DL, Burns T, Morosini P, Rothman M, Gagnon DD, Wild D, Adriaenssen I. Reliability, validity and ability to detect change of the clinician‐rated Personal and Social Performance scale in patients with acute symptoms of schizophrenia. Current Medical Research and Opinion 2009;25(2):325‐38. [PUBMED: 19192977] - PubMed
Peng 2008
-
- Peng PW, Huang MC, Tsai CJ, Pan CH, Chen CC, Chiu CC. The disparity of pharmacokinetics and prolactin study for risperidone long‐acting injection. Journal of clinical psychopharmacology 2008;28(6):726‐7. [PUBMED: 19011459] - PubMed
Přikryl 2009
-
- Přikryl R, Ustohal L, Kucerova HP, Ceskova E. Paliperidon mediated modification of cortical inhibition. Neuro Endocrinol Lett 2009;30(3):396‐399. [PUBMED: 19855366] - PubMed
Rado 2007
-
- Rado J, Dowd SM, Janicak PG. Paliperidone ER: Reformulated antipsychotic for schizophrenia Tx. Current Psychiatry 2007;6(9):75‐82.
Roser 2009
-
- Roser P, Haussleiter IS, Juckel G, Brüne M. Paliperidone in an adult patient with asperger syndrome: case report. Pharmacopsychiatry 2009;42(2):78‐9. [PUBMED: 19308885] - PubMed
Rust 1989
-
- Rust J, Golombok S. Modern Psychometrics. London: Routledge, 1989.
Schneider 2008
-
- Schneider RA, Lizer MH. Apparent seizure and atrial fibrillation associated with paliperidone. American Journal of Health‐System Pharmacy 2008;65(22):2122‐5. [PUBMED: 18997140] - PubMed
Schooler 2007
-
- Schooler N, Bossie C, Canuso C, Turkoz I, Lindenmayer JP. A "virtual" comparison of paliperidone er and risperidone ir in patients with schizophrenia. Proceedings of the International Congress on Schizophrenia Research; 2007 Mar 28‐1; Colorado Springs, Colorado. Colorado Springs, Colorado, 2007.
Schwartzer 2009
-
- Schwartzer JJ, Morrison RL, Ricci LA, Melloni RH. Paliperidone suppresses the development of the aggressive phenotype in a developmentally sensitive animal model of escalated aggression. Psychopharmacology (Berl) 2009;203(4):653‐63. [PUBMED: 19066856] - PubMed
Simpson 2006
-
- Simpson G. Paliperidone: long‐acting antipsychotic can be taken once daily. Current Psychiatry 2006;5(11):118‐20.
Simpson 2007
-
- Simpson G. Paliperidone Extended Release. CNS Drugs 2007;21(5):426‐7. - PubMed
Spina 2007
-
- Spina E, Cavallaro R. The pharmacology and safety of paliperidone extended‐release in the treatment of schizophrenia. Expert Opinion Drug Safety 2007;6(6):651‐62. [MEDLINE: ] - PubMed
Turkoz 2008
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. - PubMed
Urichuk 2008
-
- Urichuk L, Prior TI, Dursun S, Baker G. Metabolism of atypical antipsychotics: involvement of cytochrome p450 enzymes and relevance for drug‐drug interactions. Current drug metabolism 2008;9(5):410‐8. [PUBMED: 18537577] - PubMed
Vermeir 2005
-
- Vermeir M, Boom S, Naessens I, Talluri K, Eerdekens M. Absorption, metabolism and excretion of a single oral dose of 14C‐paliperidone 1 mg in healthy subjects. European Neuropsychopharmacology 2005;15(Suppl 3):S648‐9.
Vermeir 2008
-
- Vermeir M, Naessens I, Remmerie B, Mannens G, Hendrickx J, Sterkens P, Talluri K, Boom S, Eerdekens M, Osselaer N, Cleton A. Absorption, Metabolism, and Excretion of Paliperidone, a New Monoaminergic Antagonist, in Humans. Drug metabolism and Disposition 2008;36(4):769‐79. [PUBMED: 18227146] - PubMed
Vermeulen 2007
-
- Vermeulen A, Piotrovsky V, Ludwing EA. Population pharmacokinetics of risperidone and 9‐hydroxyrisperidone in patients with acute episodes associated with bipolar disorder. Journal of Pharmacokinetics and Pharmacodynamics 2007;34(2):183‐206. [PUBMED: 17136449] - PubMed
Wang 2007
-
- Wang L, Yu L, Zhang AP, Fang C, Du J, Gu NF, Qin SY, Feng GY, Li XW, Xing QH. Serum prolactin levels, plasma risperidone levels, polymorphism of cytochrome P450 2D6 and clinical response in patients with schizophrenia. Journal of Psychopharmacology 2007;21(9):837‐42. [PUBMED: 17715206] - PubMed
Wilkinson 2000
-
- Wilkinson G, Hesdon B, Wild D, Cookson R, Farina C, Sharma V, Fitzpatrick R, Jenkinson C. Self‐report quality of life measure for people with schizophrenia: the SQLS. British Journal of Psychiatry 2000;177:42‐6. [PUBMED: 10945087] - PubMed
Xia 2007
-
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, Pinfold V, Takriti Y. The Leeds Outcomes Stakeholders Survey (LOSS) Study. Proceedings of the 15th Cochrane Colloquium; 2007 Oct 23‐27; Sao Paulo. 2007.
Yang 2007
-
- Yang LP, Plosker GL. Paliperidone extended release. CNS Drugs 2007;21(5):417‐25. [PUBMED: 17447829] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

