Long-term effects of highly active antiretroviral therapy on CD4+ cell evolution among children and adolescents infected with HIV: 5 years and counting
- PMID: 18426371
- PMCID: PMC3154876
- DOI: 10.1086/587900
Long-term effects of highly active antiretroviral therapy on CD4+ cell evolution among children and adolescents infected with HIV: 5 years and counting
Abstract
Background: Lower percentages of CD4(+) T lymphocytes are associated with adverse clinical outcomes among children and adolescents infected with human immunodeficiency virus (HIV). CD4(+) lymphocyte percentage generally increases with receipt of highly active antiretroviral therapy (HAART), but long-term follow-up is required to assess whether these increases in CD4(+) cell percentage are maintained and whether they lead to normal CD4(+) cell percentages in children with severe immunosuppression.
Methods: The study population included 1236 children and adolescents perinatally infected with HIV who were enrolled in a US-based multicenter prospective cohort study (Pediatric AIDS Clinical Trials Group 219/219C) and who were not receiving HAART at study initiation. We estimated the effects of HAART, HAART with protease inhibitors, and HAART with nonnucleoside reverse-transcriptase inhibitors on CD4(+) cell percentage, using marginal structural models to account for confounding by severity.
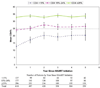
Results: Initiation of any type of HAART increased CD4(+) cell percentage by 2.34% (95% confidence interval, 1.35%-3.33%) in the first year, relative to noninitiation of HAART. The substantial increases in CD4(+) cell percentage observed after the first year of experience with these combination therapies were followed by relatively smaller increases that continued for 5 years after initiation. Although larger increases in CD4(+) cell percentage were observed among children with a greater degree of immunosuppression at baseline, the mean CD4(+) cell percentage after 5 years of HAART did not reach normal levels.
Conclusions: Our study supports the initiation of HAART in children before severe immunosuppression occurs for long-term maintenance of normal CD4(+) cell percentages. This beneficial result must be weighed against the evidence of potential adverse events associated with the prolonged use of such therapy.
Conflict of interest statement
Figures
References
-
- Lindsey JC, Hughes MD, McKinney RE, et al. Treatment-mediated changes in human immunodeficiency virus (HIV) type 1 RNA and CD4 cell counts as predictors of weight growth failure, cognitive decline, and survival in HIV-infected children. J Infect Dis. 2000;182:1385–1393. - PubMed
-
- Mofenson LM, Korelitz J, Meyer WA, 3rd, et al. The National Institute of Child Health and Human Development Intravenous Immunoglobulin Clinical Trial Study Group. The relationship between serum human immunodeficiency virus type 1 (HIV-1) RNA level, CD4 lymphocyte percent, and long-term mortality risk in HIV-1–infected children. J Infect Dis. 1997;175:1029–1038. - PubMed
-
- Palumbo PE, Raskino C, Fiscus S, et al. Predictive value of quantitative plasma HIV RNA and CD4+ lymphocyte count in HIV-infected infants and children. JAMA. 1998;279:756–761. - PubMed
-
- Mofenson LM, Harris DR, Rich K, et al. The National Institute of Child Health and Human Development Intravenous Immunoglobulin Clinical Trial Study Group. Serum HIV-1 p24 antibody, HIV-1 RNA copy number and CD4 lymphocyte percentage are independently associated with risk of mortality in HIV-1-infected children. AIDS. 1999;13:31–39. - PubMed
-
- Seage GR, 3rd, Buchacz K, Weinberg GA, et al. The Pediatric AIDS Severity Score (PASS): a multidimensional AIDS-severity adjustment for pediatric HIV infection. JAIDS. 2006;43:603–610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials