L-4F treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice
- PMID: 18426778
- PMCID: PMC2443999
- DOI: 10.1194/jlr.M800046-JLR200
L-4F treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice
Abstract
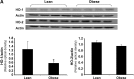
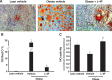
We hypothesized that the apolipoprotein mimetic peptide L-4F, which induces arterial anti-oxidative enzymes and is vasoprotective in a rat model of diabetes, would ameliorate insulin resistance and diabetes in obese mice. L-4F (2 mg/kg/d) administered to ob/ob mice for 6 weeks limited weight gain without altering food intake, decreased visceral (P < 0.02) and subcutaneous (P < 0.045) fat content, decreased plasma IL-1beta and IL-6 levels (P < 0.05) and increased insulin sensitivity, resulting in decreased glucose (P < 0.001) and insulin (P < 0.036) levels. In addition, L-4F treatment increased aortic and bone marrow heme oxygenase (HO) activity and decreased aortic and bone marrow superoxide production (P < 0.001). L-4F treatment increased serum adiponectin levels (P < 0.037) and decreased adipogenesis in mouse bone marrow (P < 0.039) and in cultures of human bone marrow-derived mesenchymal stem cells (P < 0.022). This was manifested by reduced adiposity, improved insulin sensitivity, improved glucose tolerance, increased plasma adiponectin levels, and reduced IL-1beta and IL-6 levels in obese mice. This study highlights the existence of a temporal relationship between HO-1 and adiponectin that is positively affected by L-4F in the ob/ob mouse model of diabetes, resulting in the amelioration of the deleterious effects of diabetes.
Figures










References
-
- Robertson R. P. 2004. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J. Biol. Chem. 279 42351–42354. - PubMed
-
- Bahia L., L. G. Aguiar, N. Villela, D. Bottino, A. F. Godoy-Matos, B. Geloneze, M. Tambascia, and E. Bouskela. 2006. Relationship between adipokines, inflammation, and vascular reactivity in lean controls and obese subjects with metabolic syndrome. Clinics. 61 433–440. - PubMed
-
- Kruger A. L., S. Peterson, S. Turkseven, P. M. Kaminski, F. F. Zhang, S. Quan, M. S. Wolin, and N. G. Abraham. 2005. D-4F induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes. Circulation. 111 3126–3134. - PubMed
-
- Bakkaloglu S. A., O. Soylemezoglu, N. Buyan, S. O. Oktar, T. Funahashi, H. Pasaoglu, A. H. Elhan, H. Peru, and E. Hasanoglu. 2006. Adiponectin levels and arteriosclerotic risk factors in pediatric renal transplant recipients. Pediatr. Transplant. 10 187–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

