Late endocytic multivesicular bodies intersect the chlamydial inclusion in the absence of CD63
- PMID: 18426873
- PMCID: PMC2446703
- DOI: 10.1128/IAI.00129-08
Late endocytic multivesicular bodies intersect the chlamydial inclusion in the absence of CD63
Abstract
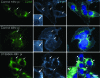
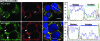
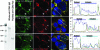
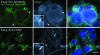
Chlamydiae are obligate intracellular bacterial pathogens that replicate solely within a membrane-bound vacuole termed an inclusion. Within the confines of the inclusion, the replicating bacteria acquire amino acids, nucleotides, and other precursors from the host cell. Trafficking from CD63-positive multivesicular bodies to the inclusion was previously identified as a novel interaction that provided essential precursors for the maintenance of a productive intracellular infection. The present study analyzes the direct delivery of resident protein and lipid constituents of multivesicular bodies to the intracellular chlamydiae. The manipulation of this trafficking pathway with an inhibitor of multivesicular body transport and the delivery of exogenous antibodies altered protein and cholesterol acquisition and delayed the maturation of the chlamydial inclusion. Although inhibitor studies and ultrastructural analyses confirmed a novel interaction between CD63-positive multivesicular bodies and the intracellular chlamydiae, neutralization with small interfering RNAs and anti-CD63 Fab fragments revealed that CD63 itself was not required for this association. These studies confirm CD63 as a constituent in multivesicular body-to-inclusion transport; however, other requisite components of these host cell compartments must control the delivery of key nutrients that are essential to intracellular bacterial development.
Figures




References
-
- Alpy, F., M. E. Stoeckel, A. Dierich, J. M. Escola, C. Wendling, M. P. Chenard, M. T. Vanier, J. Gruenberg, C. Tomasetto, and M. C. Rio. 2001. The steroidogenic acute regulatory protein homolog MLN64, a late endosomal cholesterol-binding protein. J. Biol. Chem. 2764261-4269. - PubMed
-
- Alpy, F., and C. Tomasetto. 2006. MLN64 and MENTHO, two mediators of endosomal cholesterol transport. Biochem. Soc. Trans. 34343-345. - PubMed
-
- Beatty, W. L. 2006. Trafficking from CD63-positive late endocytic multivesicular bodies is essential for intracellular development of Chlamydia trachomatis. J. Cell Sci. 119350-359. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

