Role of aromatic amino acids in receptor binding activity and subunit assembly of the cytolethal distending toxin of Aggregatibacter actinomycetemcomitans
- PMID: 18426882
- PMCID: PMC2446717
- DOI: 10.1128/IAI.00126-08
Role of aromatic amino acids in receptor binding activity and subunit assembly of the cytolethal distending toxin of Aggregatibacter actinomycetemcomitans
Abstract
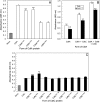
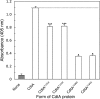
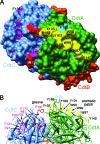
The periodontal pathogen Aggregatibacter actinomycetemcomitans produces a cytolethal distending toxin (Cdt) that inhibits the proliferation of oral epithelial cells. Structural models suggest that the CdtA and CdtC subunits of the Cdt heterotrimer form two putative lectin domains with a central groove. A region of CdtA rich in heterocyclic amino acids (aromatic patch) appears to play an important role in receptor recognition. In this study site-specific mutagenesis was used to assess the contributions of aromatic amino acids (tyrosine and phenylalanine) to receptor binding and CdtA-CdtC assembly. Predominant surface-exposed aromatic residues that are adjacent to the aromatic patch region in CdtA or are near the groove located at the junction of CdtA and CdtC were studied. Separately replacing residues Y105, Y140, Y188, and Y189 with alanine in CdtA resulted in differential effects on binding related to residue position within the aromatic region. The data indicate that an extensive receptor binding domain extends from the groove across the entire face of CdtA that is oriented 180 degrees from the CdtB subunit. Replacement of residue Y105 in CdtA and residues Y61 and F141 in CdtC, which are located in or at the periphery of the groove, inhibited toxin assembly. Taken together, these results, along with the lack of an aromatic amino acid-rich region in CdtC similar to that in CdtA, suggest that binding of the heterotoxin to its cell surface receptor is mediated predominantly by the CdtA subunit. These findings are important for developing strategies designed to block the activity of this prominent virulence factor.
Figures
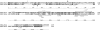




References
-
- Akifusa, S., W. Heywood, S. P. Nair, G. Stenbeck, and B. Henderson. 2005. Mechanism of internalization of the cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Microbiology 1511395-1402. - PubMed
-
- Akifusa, S., S. Poole, J. Lewthwaite, B. Henderson, and S. P. Nair. 2001. Recombinant Actinobacillus actinomycetemcomitans cytolethal distending toxin proteins are required to interact to inhibit human cell cycle progression and to stimulate human leukocyte cytokine synthesis. Infect. Immun. 695925-5930. - PMC - PubMed
-
- Ascenzi, P., P. Visca, G. Ippolito, A. Spallarossa, M. Bolognesi, and C. Montecucco. 2002. Anthrax toxin: a tripartite lethal combination. FEBS Lett. 531384-388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

