Aberrant expression of nucleostemin activates p53 and induces cell cycle arrest via inhibition of MDM2
- PMID: 18426907
- PMCID: PMC2447154
- DOI: 10.1128/MCB.01662-07
Aberrant expression of nucleostemin activates p53 and induces cell cycle arrest via inhibition of MDM2
Abstract
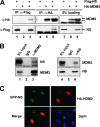
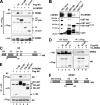
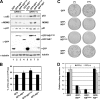
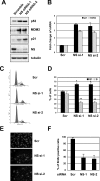
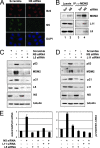
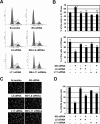
The nucleolar protein nucleostemin (NS) is essential for cell proliferation and early embryogenesis. Both depletion and overexpression of NS reduce cell proliferation. However, the mechanisms underlying this regulation are still unclear. Here, we show that NS regulates p53 activity through the inhibition of MDM2. NS binds to the central acidic domain of MDM2 and inhibits MDM2-mediated p53 ubiquitylation and degradation. Consequently, ectopic overexpression of NS activates p53, induces G(1) cell cycle arrest, and inhibits cell proliferation. Interestingly, the knockdown of NS by small interfering RNA also activates p53 and induces G(1) arrest. These effects require the ribosomal proteins L5 and L11, since the depletion of NS enhanced their interactions with MDM2 and the knockdown of L5 or L11 abrogated the NS depletion-induced p53 activation and cell cycle arrest. These results suggest that a p53-dependent cell cycle checkpoint monitors changes of cellular NS levels via the impediment of MDM2 function.
Figures

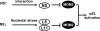
Similar articles
-
Nucleostemin: a multiplex regulator of cell-cycle progression.Trends Cell Biol. 2008 Dec;18(12):575-9. doi: 10.1016/j.tcb.2008.09.003. Epub 2008 Oct 23. Trends Cell Biol. 2008. PMID: 18951797
-
Perturbation of 60 S ribosomal biogenesis results in ribosomal protein L5- and L11-dependent p53 activation.J Biol Chem. 2010 Aug 13;285(33):25812-21. doi: 10.1074/jbc.M109.098442. Epub 2010 Jun 16. J Biol Chem. 2010. PMID: 20554519 Free PMC article.
-
PAK1IP1, a ribosomal stress-induced nucleolar protein, regulates cell proliferation via the p53-MDM2 loop.Nucleic Acids Res. 2011 Mar;39(6):2234-48. doi: 10.1093/nar/gkq1117. Epub 2010 Nov 21. Nucleic Acids Res. 2011. PMID: 21097889 Free PMC article.
-
Putting a finger on growth surveillance: insight into MDM2 zinc finger-ribosomal protein interactions.Cell Cycle. 2007 Feb 15;6(4):434-7. doi: 10.4161/cc.6.4.3861. Epub 2007 Feb 18. Cell Cycle. 2007. PMID: 17329973 Review.
-
A new PICTure of nucleolar stress.Cancer Sci. 2012 Apr;103(4):632-7. doi: 10.1111/j.1349-7006.2012.02219.x. Epub 2012 Mar 8. Cancer Sci. 2012. PMID: 22320853 Free PMC article. Review.
Cited by
-
Crucial role of TSC-22 in preventing the proteasomal degradation of p53 in cervical cancer.PLoS One. 2012;7(8):e42006. doi: 10.1371/journal.pone.0042006. Epub 2012 Aug 1. PLoS One. 2012. PMID: 22870275 Free PMC article.
-
Targeting the ubiquitin-mediated proteasome degradation of p53 for cancer therapy.Curr Pharm Des. 2013;19(18):3248-62. doi: 10.2174/1381612811319180009. Curr Pharm Des. 2013. PMID: 23151129 Free PMC article. Review.
-
Interplay between human nucleolar GNL1 and RPS20 is critical to modulate cell proliferation.Sci Rep. 2018 Jul 30;8(1):11421. doi: 10.1038/s41598-018-29802-y. Sci Rep. 2018. PMID: 30061673 Free PMC article.
-
Human embryonic stem cell-derived neural crest model unveils CD55 as a cancer stem cell regulator for therapeutic targeting in MYCN-amplified neuroblastoma.Neuro Oncol. 2022 Jun 1;24(6):872-885. doi: 10.1093/neuonc/noab241. Neuro Oncol. 2022. PMID: 34655293 Free PMC article.
-
Ribosomal protein L11 recruits miR-24/miRISC to repress c-Myc expression in response to ribosomal stress.Mol Cell Biol. 2011 Oct;31(19):4007-21. doi: 10.1128/MCB.05810-11. Epub 2011 Aug 1. Mol Cell Biol. 2011. PMID: 21807902 Free PMC article.
References
-
- Banin, S., L. Moyal, S. Shieh, Y. Taya, C. W. Anderson, L. Chessa, N. I. Smorodinsky, C. Prives, Y. Reiss, Y. Shiloh, and Y. Ziv. 1998. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 2811674-1677. - PubMed
-
- Canman, C. E., D. S. Lim, K. A. Cimprich, Y. Taya, K. Tamai, K. Sakaguchi, E. Appella, M. B. Kastan, and J. D. Siliciano. 1998. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 2811677-1679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
