Intracellular signaling in primary sensory neurons and persistent pain
- PMID: 18427980
- PMCID: PMC2570619
- DOI: 10.1007/s11064-008-9711-z
Intracellular signaling in primary sensory neurons and persistent pain
Abstract
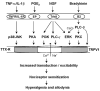
During evolution, living organisms develop a specialized apparatus called nociceptors to sense their environment and avoid hazardous situations. Intense stimulation of high threshold C- and Adelta-fibers of nociceptive primary sensory neurons will elicit pain, which is acute and protective under normal conditions. A further evolution of the early pain system results in the development of nociceptor sensitization under injury or disease conditions, leading to enhanced pain states. This sensitization in the peripheral nervous system is also called peripheral sensitization, as compared to its counterpart, central sensitization. Inflammatory mediators such as proinflammatory cytokines (TNF-alpha, IL-1beta), PGE(2), bradykinin, and NGF increase the sensitivity and excitability of nociceptors by enhancing the activity of pronociceptive receptors and ion channels (e.g., TRPV1 and Na(v)1.8). We will review the evidence demonstrating that activation of multiple intracellular signal pathways such as MAPK pathways in primary sensory neurons results in the induction and maintenance of peripheral sensitization and produces persistent pain. Targeting the critical signaling pathways in the periphery will tackle pain at the source.
Figures

References
-
- Rau KK, Jiang N, Johnson RD, et al. Heat sensitization in skin and muscle nociceptors expressing distinct combinations of TRPV1 and TRPV2 protein. J Neurophysiol. 2007;97:2651–2662. - PubMed
-
- Kwan KY, Allchorne AJ, Vollrath MA, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. - PubMed
-
- Caterina MJ, Rosen TA, Tominaga M, et al. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

